- Department of Neurosurgery, National Hospital Organization (NHO) Osaka National Hospital, Osaka, Japan
- Department of Neurosurgery, Osaka University Graduate School of Medicine, Suita, Japan
- Department of Biomedical Research and Innovation, Institute for Clinical Research, National Hospital Organization (NHO) Osaka National Hospital, Osaka, Japan.
Correspondence Address:
Nobuyuki Izutsu, Department of Neurosurgery, NHO Osaka National Hospital, Osaka, Japan.
DOI:10.25259/SNI_942_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yosuke Fujimi1, Tomohiko Ozaki2, Nobuyuki Izutsu1, Shin Nakajima1, Yonehiro Kanemura1,3, Tomoki Kidani1, Saki Kawamoto1, Naoki Nishizawa1, Koji Kobayashi1, Toshiyuki Fujinaka1. Fungal symptomatic intracranial aneurysm treated with a flow diverting stent: A case report. 23-Feb-2024;15:58
How to cite this URL: Yosuke Fujimi1, Tomohiko Ozaki2, Nobuyuki Izutsu1, Shin Nakajima1, Yonehiro Kanemura1,3, Tomoki Kidani1, Saki Kawamoto1, Naoki Nishizawa1, Koji Kobayashi1, Toshiyuki Fujinaka1. Fungal symptomatic intracranial aneurysm treated with a flow diverting stent: A case report. 23-Feb-2024;15:58. Available from: https://surgicalneurologyint.com/surgicalint-articles/12762/
Abstract
Background: Intracranial infectious aneurysms (IIAs) are very rare, and fungal aneurysms are infrequently reported. We report a case of an unruptured IIA caused by fungal rhinosinusitis and treated with a flow-diverting stent.
Case Description: An 81-year-old woman visited the ophthalmology department with impaired eye movement and ptosis and was placed under follow-up. A week later, she also developed a headache; magnetic resonance angiography revealed an aneurysm measuring 2 mm in the C4 portion of the right internal carotid artery. A 3-week follow-up with contrast-enhanced magnetic resonance imaging showed an increase in its size to 10 mm, and a contrast lesion was observed surrounding the right cavernous sinus. The patient started treatment with voriconazole and steroids on the same day. Ten weeks later, despite improvements in inflammation, the size of the aneurysm was unchanged; we, therefore, treated the aneurysm with a flow-diverting stent. Oculomotor nerve palsy improved, and the patient was discharged to a rehabilitation hospital 28 days after the placement, with a modified Rankin Scale of 4. A 1-year follow-up angiogram showed a partial decrease in the size of the aneurysm, with an O’Kelly-Marotta grading scale of B3.
Conclusion: IIAs grow rapidly, and the risk of rupture is high due to the weakening of the aneurysmal wall. To reduce the risks of rupture and recurrence after treatment, the infection should be treated before inserting a flow-diverting stent. Flow-diverting stent placement may be an effective treatment for IIA once the original infection has been cured.
Keywords: Flow diverting stent, Intracranial fungal aneurysm, Intracranial infectious aneurysm
INTRODUCTION
Intracranial infectious aneurysm (IIA) is a rare disease[
CASE DESCRIPTION
An 80-year-old woman with a medical history of cerebral infarction, ovarian cancer, and breast cancer noted sudden right eye ptosis and abduction. She visited her local ophthalmology department for a check-up and was placed under follow-up. One week later, she had a gradually worsening headache and was taken by ambulance to a local hospital. Magnetic resonance angiography revealed a 2-mm aneurysm in the C4 portion of the right internal carotid artery (ICA); however, the relationship between the aneurysm and her symptoms was unclear [
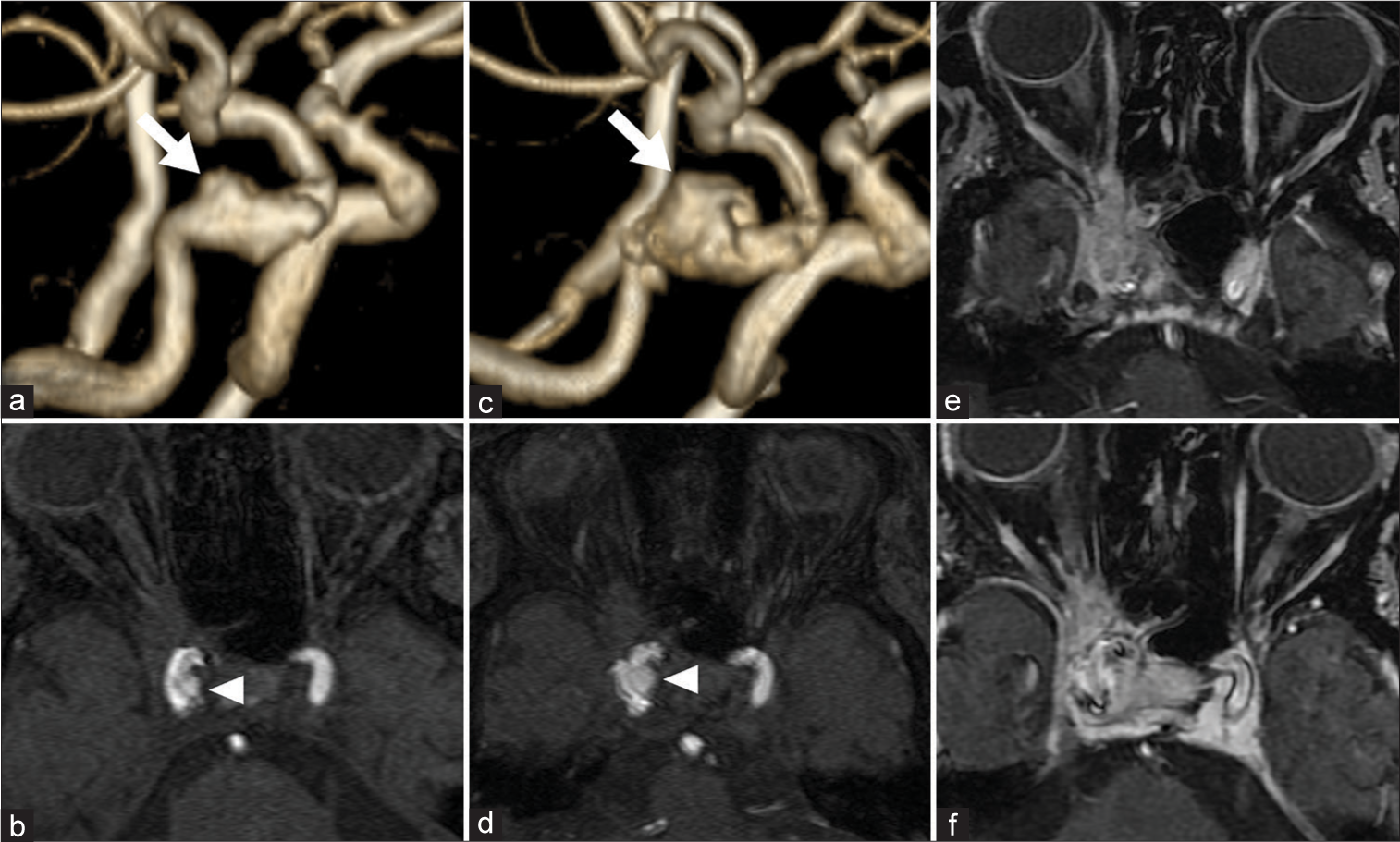
Figure 1:
(a and b) First magnetic resonance angiography of the aneurysm, with a size of approximately 2 mm (arrow and arrowhead). (c and d) The aneurysm size increased to 10 mm in 2 weeks (arrow and arrowhead). (e and f) Contrast-enhanced magnetic resonance imaging demonstrates a contrast-enhanced lesion from the right cavernous sinus to the orbital apex, which communicates with the sphenoid sinus.
Moreover, the cavernous portion of the right ICA was dilated with wall irregularities caused by vascular invasion [
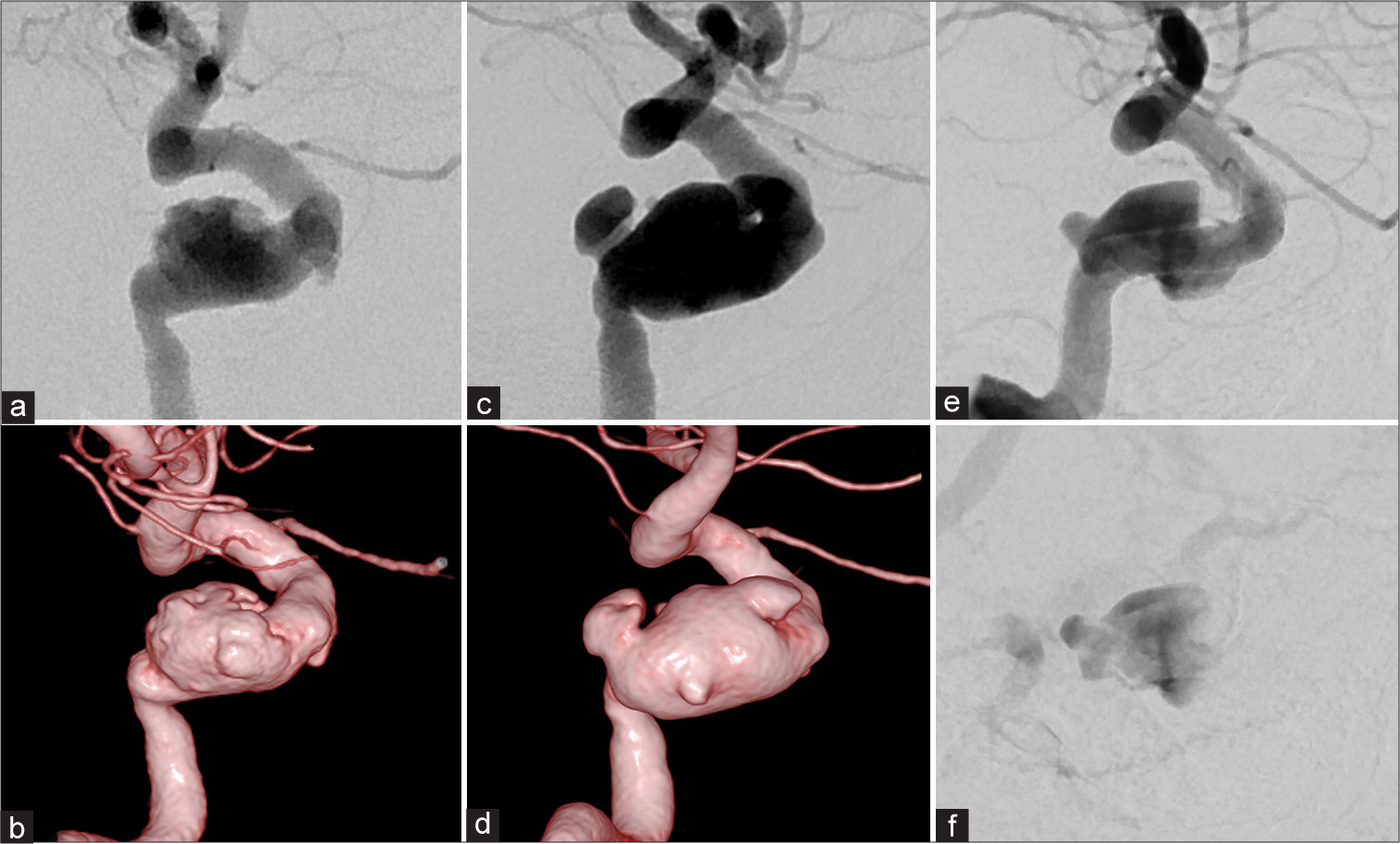
Figure 2:
(a and b) First angiogram demonstrating the aneurysm in the cavernous portion of the right internal carotid artery with wall irregularities. (c and d) Angiogram after six weeks of medical treatment exhibiting the aneurysm dilatation and shape change. (e and f) Arterial phase and venous phase of angiogram one year after flow diverting stent placement. O’Kelly-Marotta grading scale: B3.
DISCUSSION
IIA often occurs in patients with conditions such as poorly controlled diabetes mellitus, leukemia, ongoing chemotherapy, or steroid therapy.[
Infective endocarditis is the most common cause of IIA[
Different IIAs are thought to have distinct characteristic locations. For example, intracranial bacterial cerebral aneurysms are often caused by infective endocarditis[
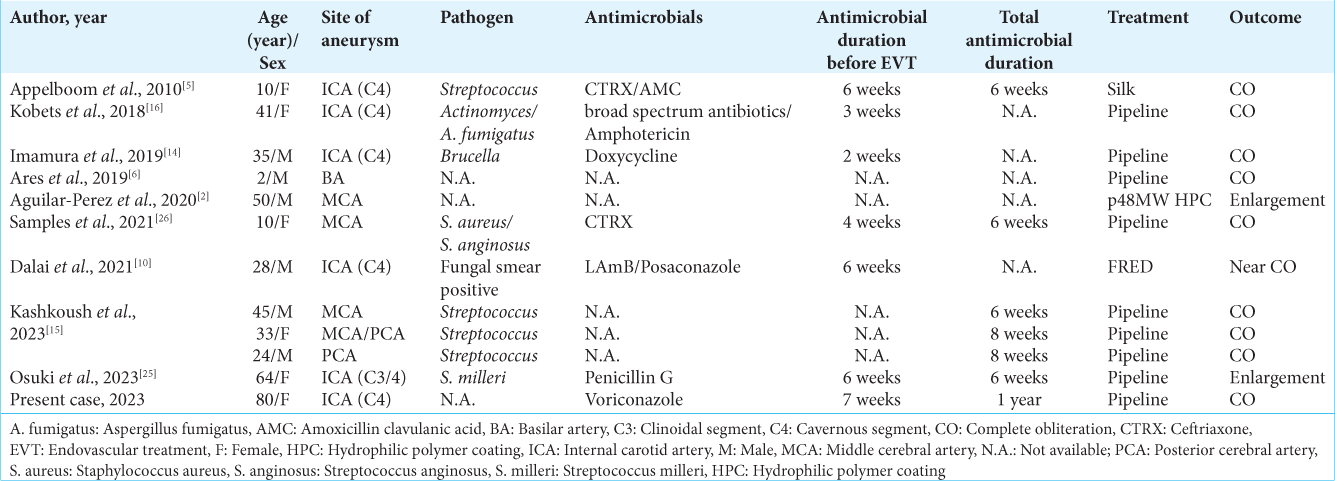
There is no established duration of antifungal medical treatment for intracranial fungal aneurysms. According to a retrospective cohort study of the use of antifungals and outcomes of invasive aspergillosis or mucormycosis, the average duration of antifungal treatment was 41 days.[
IIAs rapidly grow and deform, thus inducing destruction and necrosis of the surrounding organs.[
CONCLUSION
Flow-diverting stent placement may be an effective treatment for symptomatic intracranial fungal aneurysms once the original infection has been cured.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abdel-Tawab M, Abdeltawab AK, Abdelmonem M, Moubark MA, Taha MA, Morsy A. Efficacy and safety of flow diverters in posterior circulation aneurysms and comparison with their efficacy in anterior circulation aneurysms: A systematic review and meta-analysis. Interv Neuroradiol. 2021. 27: 609-21
2. Aguilar-Perez M, Hellstern V, AlMatter M, Wendl C, Bazner H, Ganslandt O. The p48 flow modulation device with hydrophilic polymer coating (HPC) for the treatment of acutely ruptured aneurysms: Early clinical experience using single antiplatelet therapy. Cardiovasc Intervent Radiol. 2020. 43: 740-8
3. Alawieh A, Chaudry MI, Turner RD, Turk AS, Spiotta AM. Infectious intracranial aneurysms: A systematic review of epidemiology, management, and outcomes. J Neurointerv Surg. 2018. 10: 708-16
4. Allen LM, Fowler AM, Walker C, Derdeyn CP, Nguyen BV, Hasso AN. Retrospective review of cerebral mycotic aneurysms in 26 patients: Focus on treatment in strongly immunocompromised patients with a brief literature review. AJNR Am J Neuroradiol. 2013. 34: 823-7
5. Appelboom G, Kadri K, Hassan F, Leclerc X. Infectious aneurysm of the cavernous carotid artery in a child treated with a new-generation of flow-diverting stent graft: Case report. Neurosurgery. 2010. 66: E623-4 discussion E4
6. Ares WJ, Tonetti DA, Greene S, Sharma MS, Xavier F, Jankowitz BT. Pipeline embolization of an infectious basilar artery aneurysm in a 2-year-old child: Case report, discussion of the literature and perioperative considerations. Oper Neurosurg (Hagerstown). 2019. 17: E224-8
7. Azar MM, Assi R, Patel N, Malinis MF. Fungal mycotic aneurysm of the internal carotid artery associated with sphenoid sinusitis in an immunocompromised patient: A case report and review of the literature. Mycopathologia. 2016. 181: 425-33
8. Chapot R, Houdart E, Saint-Maurice JP, Aymard A, Mounayer C, Lot G. Endovascular treatment of cerebral mycotic aneurysms. Radiology. 2002. 222: 389-96
9. Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018. 33: 463-79.e10
10. Dalai S, Datla AV, Francis AA, Dannana NK, Parappil H. Endovascular management of mucormycotic aneurysms of the internal carotid artery in post-COVID-19 patients. Cureus. 2021. 13: e20812
11. Fang YB, Lin A, Kostynskyy A, Agid R, Tymianski M, Radovanovic I. Endovascular treatment of intracranial vertebrobasilar artery dissecting aneurysms: Parent artery occlusion versus flow diverter. Eur J Radiol. 2018. 99: 68-75
12. Hiramatsu R, Yagi R, Kameda M, Nonoguchi N, Furuse M, Kawabata S. Treatment outcomes of 94 cases of pipeline embolization device in a single center: Predictive factors of incomplete aneurysm occlusion. J Neuroendovasc Ther. 2023. 17: 217-23
13. Hot A, Mazighi M, Lecuit M, Poiree S, Viard JP, Loulergue P. Fungal internal carotid artery aneurysms: Successful embolization of an Aspergillus associated case and review. Clin Infect Dis. 2007. 45: e156-61
14. Imamura H, Sakai N, Alexander MJ. Flow-diverter stenting of intracavernous internal carotid artery mycotic aneurysm. J Stroke Cerebrovasc Dis. 2019. 28: e81-2
15. Kashkoush A, El-Abtah ME, Achey R, Hussain MS, Toth G, Moore NZ. Flow diversion as destination treatment of intracranial mycotic aneurysms: A retrospective case series. Oper Neurosurg (Hagerstown). 2023. 24: 492-8
16. Kobets AJ, Scoco A, Nakhla J, Brook AL, Kinon MD, Baxi N. Flow-diverting stents for the obliteration of symptomatic, infectious cavernous carotid artery aneurysms. Oper Neurosurg (Hagerstown). 2018. 14: 681-5
17. Koiso T, Komatsu Y, Matsumaru Y, Ishikawa E. Difficulty of diagnosing a mucor-induced aneurysm arising in segment P4 of the posterior cerebral artery-A case report. Surg Neurol Int. 2022. 13: 111
18. Komatsu Y, Narushima K, Kobayashi E, Tomono Y, Nose T. Aspergillus mycotic aneurysm--case report. Neurol Med Chir (Tokyo). 1991. 31: 346-50
19. Maragkos GA, Ascanio LC, Salem MM, Gopakumar S, Gomez-Paz S, Enriquez-Marulanda A. Predictive factors of incomplete aneurysm occlusion after endovascular treatment with the Pipeline embolization device. J Neurosurg. 2019. 132: 1598-605
20. Matsubara N, Miyachi S, Izumi T, Yamanouchi T, Asai T, Ota K. Results and current trends of multimodality treatment for infectious intracranial aneurysms. Neurol Med Chir (Tokyo). 2015. 55: 155-62
21. Miyachi S, Ohnishi H, Hiramatsu R, Izumi T, Matsubara N, Kuroiwa T. Innovations in endovascular treatment strategies for large carotid cavernous aneurysms-the safety and efficacy of a flow diverter. J Stroke Cerebrovasc Dis. 2017. 26: 1071-80
22. Nakahara I, Taha MM, Higashi T, Iwamuro Y, Iwaasa M, Watanabe Y. Different modalities of treatment of intracranial mycotic aneurysms: Report of 4 cases. Surg Neurol. 2006. 66: 405-9 discussion 9-10
23. Nishi H, Ishii A, Satow T, Iihara K, Sakai N. Parent artery occlusion for unruptured cerebral aneurysms: Results of the Japanese registry of neuroendovascular therapy 3. Neurol Med Chir (Tokyo). 2019. 59: 1-9
24. Okawa S, Hanazono A, Sugawara M, Takahashi S, Yanagiwsawa T, Mizoi K. Cavernous sinus thrombosis with infectious intracranial aneurysms: A case report. Jpn J Stroke. 2010. 32: 307-13
25. Osuki T, Ikeda H, Uezato M, Kinosada M, Kurosaki Y, Chin M. De novo expansion formation in the outer curvature of the internal carotid artery after flow diverter deployment for an infectious cavernous carotid aneurysm: Illustrative case. J Neurosurg Case Lessons. 2023. 5: CASE23124
26. Samples DC, Ravindra VM, Thoms DJ, Tarasiewicz I, Grandhi R. Successful flow diversion treatment of ruptured infectious middle cerebral artery aneurysms with the use of Pipeline Flex with Shield technology. Interv Neuroradiol. 2021. 27: 225-9
27. Stull K, Esterberg E, Ajmera M, Candrilli S, Kitt TM, Spalding JR. Use of antifungals and outcomes among inpatients at risk of invasive aspergillosis or mucormycosis in the USA: A retrospective cohort study. Infect Dis Ther. 2019. 8: 641-55
28. Terada E, Nishida T, Fujita Y, Maeda Y, Hayama M, Takagaki M. Percutaneous transluminal angioplasty and stenting for progressive intracranial carotid artery stenosis secondary to invasive sphenoid sinus aspergillosis: A case report. NMC Case Rep J. 2023. 10: 215-20
29. Tokuda T, Ono Y, Nishiya H, Aoki M, Yamanouchi S, Kunii O. An autopsy case of fungal (Mucor) cerebral aneurysm. Kansenshogaku Zasshi. 1995. 69: 438-43
30. Tokunaga H, Kamada K, Hoshida T, Kawaguchi S, Uranishi R, Shiomi K. The treatment for the bacterial aneurysm. Surg Cereb Stroke. 1991. 19: 476-81
31. Yamaguchi J, Kawabata T, Motomura A, Hatano N, Seki Y. Fungal internal carotid artery aneurysm treated by trapping and high-flow bypass: A case report and literature review. Neurol Med Chir (Tokyo). 2016. 56: 89-94