- Department of Neurosurgery, Fundación Clínica Shaio, Bogotá, Colombia.
- Department of Research, Fundación Clínica Shaio, Bogotá, Colombia.
Correspondence Address:
Oscar I. Molina-Romero, Department of Neurosurgery, Fundación Clínica Shaio, Bogotá, Colombia.
DOI:10.25259/SNI_679_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Oscar I. Molina-Romero1, Andrés Segura-Hernandez1, Andrés Fonnegra-Caballero1, Juan Carlos Diez-Palma1, Fabian Cortés-Muñoz2, Julio Roberto Fonnegra-Pardo1. Gamma Knife radiosurgery – 12 years of experience in a high-complexity center of a middle-income country. 16-Dec-2022;13:582
How to cite this URL: Oscar I. Molina-Romero1, Andrés Segura-Hernandez1, Andrés Fonnegra-Caballero1, Juan Carlos Diez-Palma1, Fabian Cortés-Muñoz2, Julio Roberto Fonnegra-Pardo1. Gamma Knife radiosurgery – 12 years of experience in a high-complexity center of a middle-income country. 16-Dec-2022;13:582. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12059
Abstract
Background: Gamma Knife radiosurgery (GKR) is a technique that consists of the release of a high dose of ionizing radiation onto a therapeutic target, which has been previously delimited. This technique was described by Lars Leksell and Borje Larsson in 1951. In Colombia, there is only one GKR unit functioning machine nowadays. The objective of this study is to describe the institutional experience of a single institution with Gamma Knife Perfexion over 12 years.
Methods: We conducted a retrospective observational study. A total of 1906 medical records, taken from the period between May 4, 2010, and May 4, 2022, were included in the study. Descriptive analysis was performed through STATA 17 as statistic tool. Measures of central tendency were calculated depending on the distribution of the continuous data and proportions were taken into account in the case of qualitative variables.
Results: A total of 1906 procedures were performed. Patients from 1 year to 99 years old were treated, with a median age of 51 years. The most frequent diagnoses were meningioma (20.8%), arteriovenous malformation (AVM) (17%), vestibular schwannoma (15.6%), metastases (9.81%), and trigeminal neuralgia (9.12%). At 3-year posttreatment, in meningiomas, tumor size stability was observed in 57.3%, size decrease in 36%, and disappearance in 1.3%. In AVM, complete obliteration of the lesion was described in 36.8% and a decrease in size in 52.6%. Intracranial hemorrhage occurred in 5.2% during the follow-up period and 3.5% of all treated patients required a new procedure due to residual malformation. In vestibular schwannomas, tumor size remained stable in 62.2% and decreased in 28.8%. No new cases of facial paralysis after the procedure were described. At 1-year posttreatment, in metastasis, the size of the lesions remained stable in 40% of the patients, decreased in 47.5%, and disappeared in 2.5%. In trigeminal neuralgia, 88.4% of patients had pain relief and recurrences occurred in 16.6%. Acute complications were generally uncommon, the main ones being headache, pain at frame fixation points, and nausea.
Conclusion: Our experience suggests that GKR is a noninvasive procedure with a broad spectrum of clinical applications, low frequency of complications, feasible, with good enough control size of tumor and vascular lesions in images, and good clinical results in the medium and long term.
Keywords: Arteriovenous malformation, Brain neoplasms, Gamma Knife radiosurgery, Stereotactic radiosurgery, Trigeminal neuralgia
INTRODUCTION
Gamma Knife radiosurgery (GKR) is a technique that consists of the release of a high dose of ionizing radiation onto a therapeutic target, which has been previously located and delimited through stereotaxy.[
After being used for more than a decade at the Karolinska Institute in Sweden, more units were installed in other countries such as Argentina, England, and the United States.[
The Gamma Knife Perfexion uses 192 cobalt-60 sources whose radiation shots are emitted toward a previously defined intracranial point, through three basic collimators distributed centrifugally in eight segments.[
Multiple publications have reported institutional experiences in the management of specific pathologies with GKR. However, few institutions have reported their general experience.[
MATERIALS AND METHODS
We conducted a retrospective observational study. The data for this study were from Fundación Clínica Shaio, a fourth-level hospital in Bogotá, Colombia. We conducted this research with the approval of the Institution’s Ethics Committee. A total of 1906 medical records, taken from the period between May 4, 2010, and May 4, 2022, were included in the study. For the follow-up data, three periods were taken into account: (1) between the 1st week and the 1st month posttreatment to evaluate acute complications, (2) 1-year posttreatment in patients with metastases and trigeminal neuralgia, and (3) 3-year posttreatment in the other three most frequent diagnoses (meningioma, arteriovenous malformation [AVM], and vestibular schwannoma).
The data were analyzed using the statistical program STATA 17.0. For quantitative variables, normality in the distribution of the data was verified by graphs, the Shapiro–Wilk test, and kurtosis. According to the results, measures of central tendency were applied, mainly median with interquartile range (IQR), and mean with standard deviation (SD). In the case of qualitative variables, proportions were calculated.
Procedure
A rigid frame is placed on the patient’s head under local anesthesia or with the assistance of general anesthesia in the case of pediatric patients younger than 12 years, patients with claustrophobia, or with a history of psychiatric disorders that prevent the realization of the procedure. Once fixed to the skull, the stereotactic frame helps to establish stereotactic coordinates in the intracranial space, to pinpoint the therapeutic target.
With the frame in place, high-resolution neuroimaging is performed, mainly CT, 1 mm thin-slice MRI of the brain, and in the case of AVM, cerebral angiography. Based on these images, treatment planning is carried out in the GammaPlan software using a coordinate system that allows locating and delimiting the intracranial lesion. The patient enters the radiosurgery machine which emits gamma radiation with high precision to the intracranial lesion, minimizing the irradiation of adjacent structures and with a <1 mm margin of error. The dose of radiation emitted is decided according to the type of lesion, location, size, and history of previous management by the neurosurgery service and the medical physicists, which during the procedure supervise and control the radiation release.
In the case of large lesions with increased risk of cerebral edema due to radiation, or in patients whose neurological functions such as hearing must be preserved, we do the procedure in fractions, generally in two or three. This allows us to treat lesions using lower doses per fraction [
RESULTS
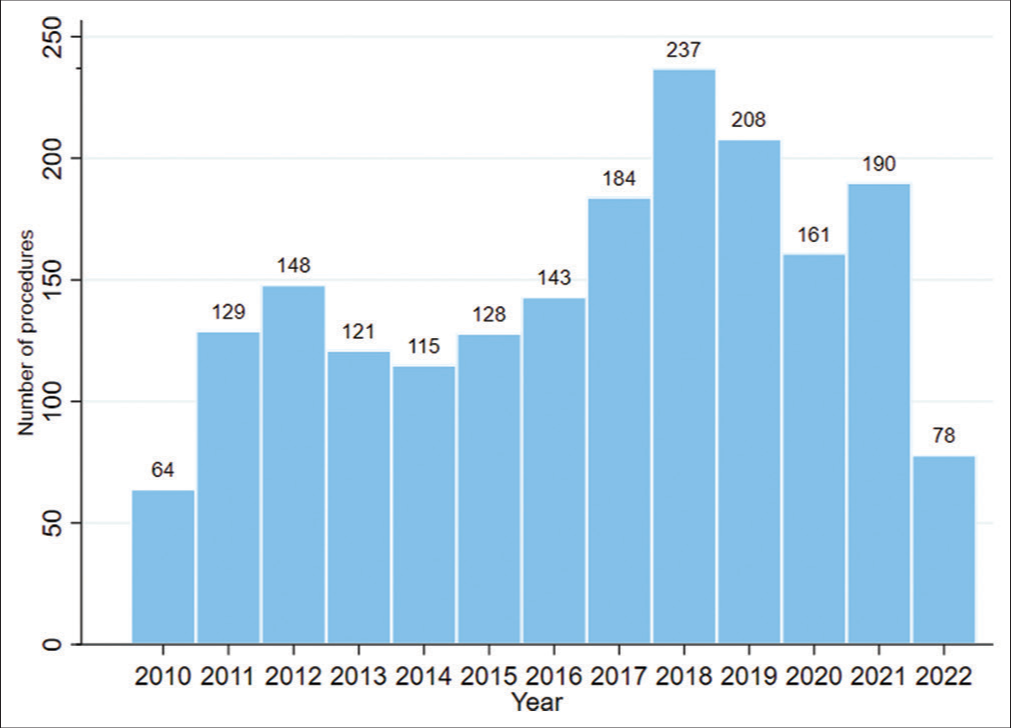
Between May 4, 2010, and May 4, 2022, 1774 patients were treated with Gamma Knife Perfexion at our institution [
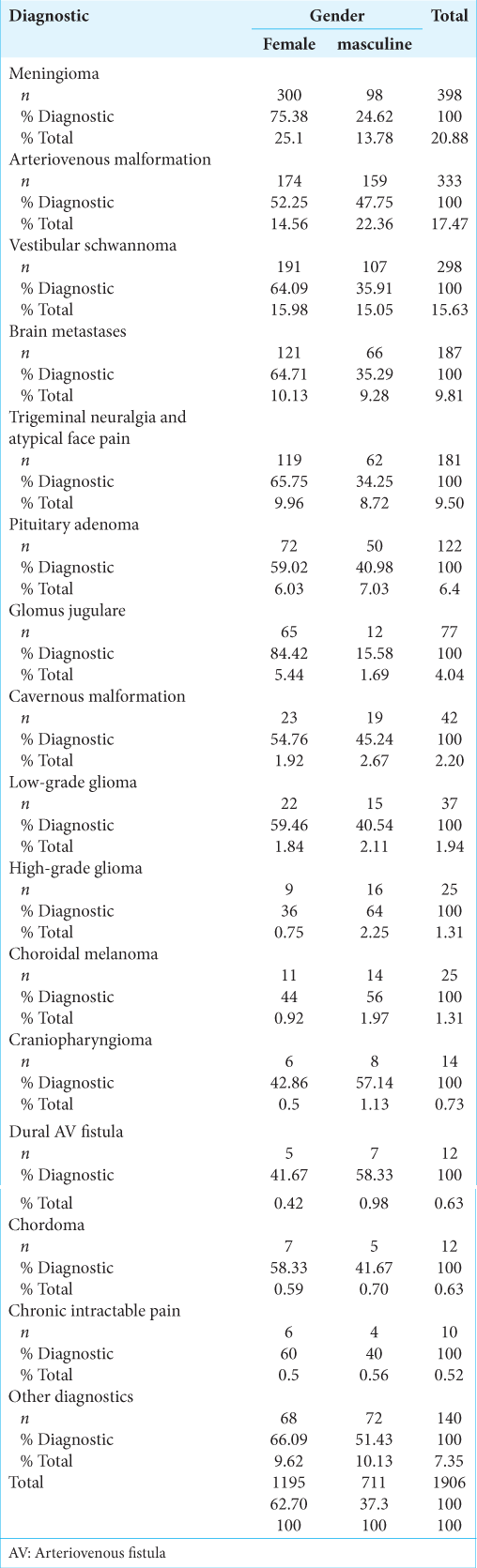
The most frequent diagnoses [
Meningiomas
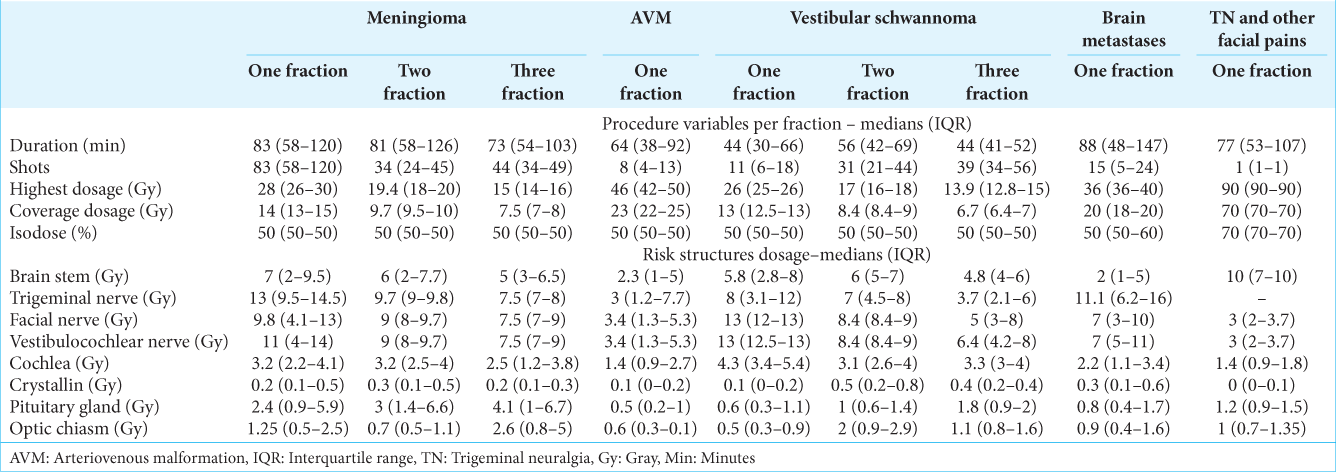
398 procedures were performed, corresponding to 20.88% of the total. Three hundred procedures (75.3%) were performed on women and 98 (24.6%) were performed on men. In total, 387 patients were treated. The mean coverage dosage was 14 Gy. The median age was 54.5 years (IQR 44–64). About 40% of the patients had a history of previous surgical management. In 377 procedures (94.7%), single lesions were treated, while in 21 procedures (5.2%) between two and six lesions were treated per procedure, all of them in patients diagnosed with neurofibromatosis type 2. A total of 432 lesions were treated, of which 66.6% were skull base meningiomas, 12.5% convexity meningiomas, 12% midline meningiomas, 5.5% posterior fossa meningiomas, 2.3% optic nerve sheath meningiomas, and 0.93% orbital meningiomas. The median volume of the lesions was 6.55 cc (IQR 3.1–12.6). Of the 398 procedures, 57.8% were performed in a single fraction, whereas 42.2% were divided into 2–5 fractions. The median hospital stay was 5.42 h (IQR 3.3–29.9). The treatment data are shown in
At 3 years posttreatment, follow-up data were obtained from 75 patients (18.8% of the total), with a median follow-up of 36.2 months (IQR 27.9–38.8). The size of the tumor remained stable in 57.3%, in 36%, the size decreased [
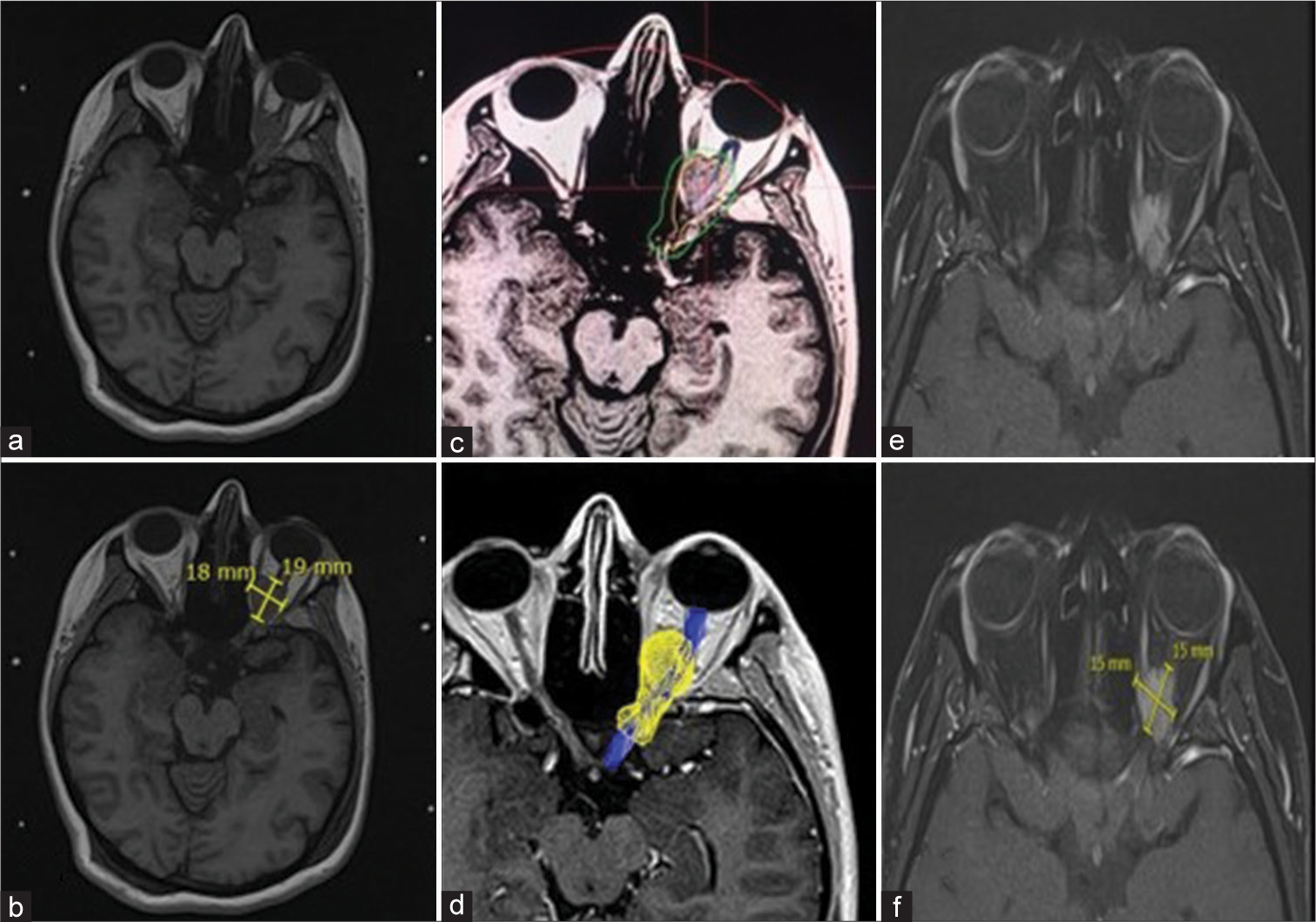
Figure 3:
A 32-year-old woman who presented left fronto-orbital headache, blurred vision, and floaters in the left eye. (a and b) Magnetic resonance imaging (MRI) shows an infiltrating mass of the left orbital apex suggestive of a meningioma that surrounds the optic nerve and generates local compression. (c and d) MRI at time of Gamma Knife radiosurgery. The optic nerve (blue) was identified and the tumor was precisely delimited (yellow). To conserve optic nerve function, fractional treatment with two sessions was performed, coverage dose of 9.7 Gy and isodose of 50% for each session were released. (e and f) Orbital MRI 3 years after treatment. A smaller tumor is observed with no radiological signs of compression on the optic nerve. Clinically, the patient with an improvement in visual acuity and floaters.
AVMs
333 procedures (17.4%) were carried out, 52.2% on women and 47.7% on men. A total of 312 patients were treated. The median age was 28 years (IQR 19–41). The mean coverage dosage was 23 Gy. Of the total lesions treated, 76.8% were supratentorial, 13.2% basal ganglia, and 9.9% posterior fossa. About 61.2% of the patients had a history of previous intracranial bleeding. About 17.9% had a history of previous surgical management, mainly drainage of hematomas, placement of external ventriculostomy, ventriculoperitoneal shunt, and endoscopic third ventriculostomy. On the other hand, 56.7% had a history of previous embolization, with a median of two embolizations (IQR 1–3). The median volume of the lesions was 3 cc (IQR 1–6.3). All procedures were performed in a single fraction and the median hospital stay was 4.01 h (IQR 2.98–5.56). Treatment data are shown in
At 3 years posttreatment, follow-up data were obtained from 38 patients, 12.1% of the total, with a mean follow-up of 33.5 months (SD 7.8). In 10.5%, stability of the size of the lesion was documented with magnetic resonance and angiography, in 52.6%, the size decreased, and in 36.8%, complete obliteration of the lesion was described [
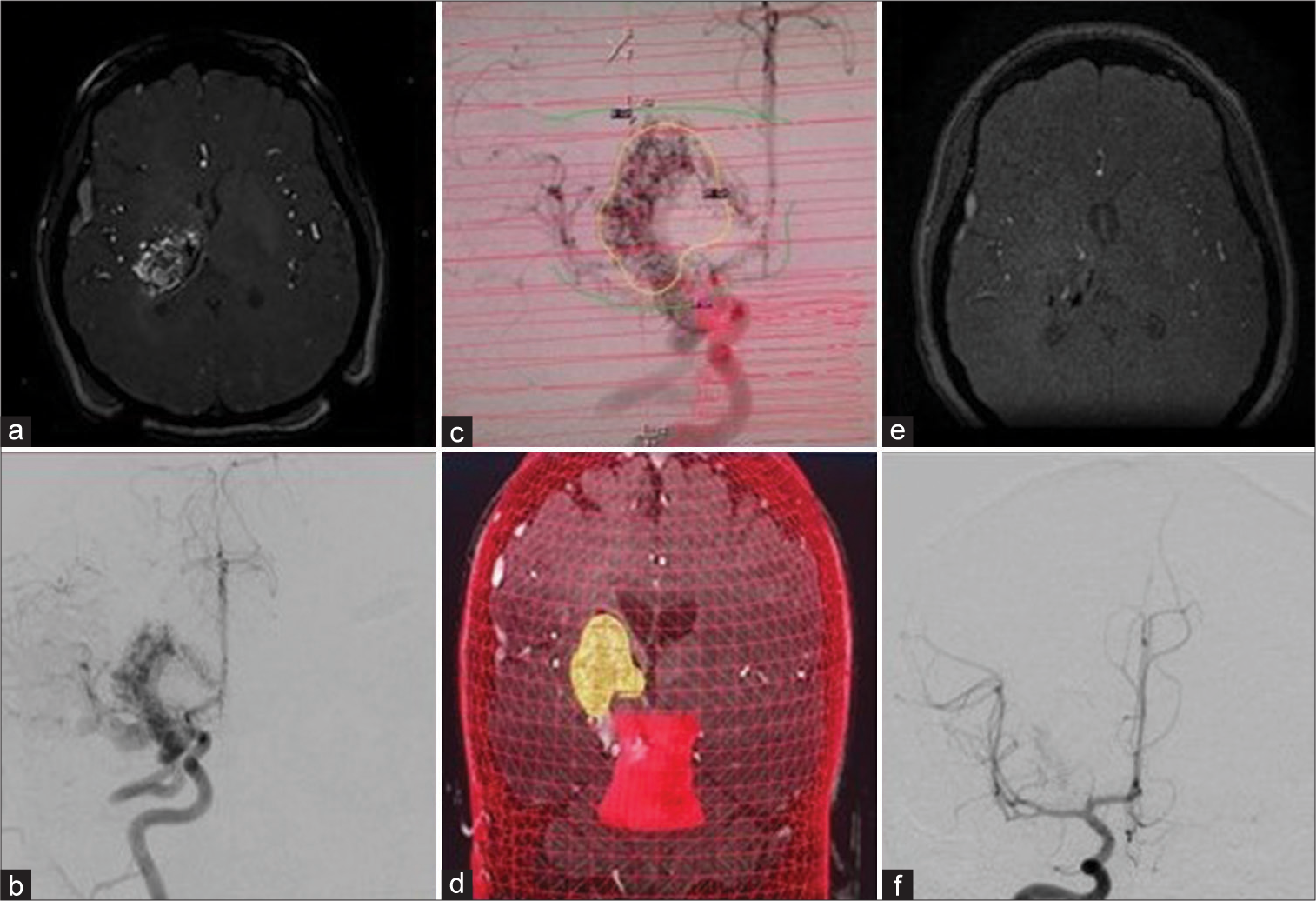
Figure 4:
A 37-year-old woman who presented intracerebral hemorrhage due to a ruptured arteriovenous malformation (AVM) in the right basal ganglia region. She required external ventriculostomy and embolization, which achieved occlusion of 10% of the malformation. (a) Magnetic resonance imaging (MRI) in TOF sequence shows aberrant blood vessels at the level of the right thalamus, with embolization material inside. (b) Cerebral angiography shows an AVM fed by branches of the right middle and posterior cerebral arteries with superficial and deep venous drainage and a volume of 9 cc. (c and d) Cerebral angiography at time of radiosurgery. In yellow, the AVM was delimited, which received 21 Gy with 50% isodose. In red, the brainstem is observed, which received 6 Gy. e: MRI in TOF sequence 3 posttreatment, in which disappearance of the AVM is observed. f: Cerebral angiography 3 years posttreatment shows a marked reduction in AVM size.
Vestibular schwannoma
298 procedures (15.63%) were performed, 64.1% on women and 35.9% on men. A total of 293 patients were treated, and five patients were treated more than once due to the presence of a contralateral lesion in the context of neurofibromatosis type 2. The mean coverage dosage was 13 Gy. The median age was 51 years (IQR 36–63). Sixty-seven patients (22.8%) had a history of previous surgical management. About 20.4% of the vestibular schwannomas treated were Koos I, 41.2% Koos II, 24.1% Koos III, and 14% Koos IV. In 50.3% of the procedures, the lesions were right, in 47.9% left, and in 1.6% bilateral. The median volume of the lesions was 1.7 cc (IQR 0.5–4.8). Partial hearing loss was present in 78.8% of patients, tinnitus in 50.1%, vertigo in 48.1%, facial paralysis in 15.3%, total deafness in 10.2%, and in 2.3% of patients were incidental findings. Of the patients with facial paralysis, 82.2% had a history of previous surgical management, while in patients with total deafness, 73.3% shared the same background. About 80.2% of the procedures were performed in a single fraction, while 19.8% were performed in two and three fractions. The median hospital stay was 4.56 h (2.9– 22.9). The treatment data are shown in
At 3 years posttreatment, follow-up data were obtained from 45 patients, 15.3% of the total, with a median follow-up of 30.4 months (IQR 25–36.7). In 62.2% of the cases, the size of the tumor remained stable, in 28.8%, it decreased, in 6.6%, it increased (these patients with Koos IV), and in 2.2%, there was pseudo growth due to edema. Only in one case (2.2%) in wich tumor growth was reported after GKR, additional surgical management was required. 82.2% of the patients reported improvement in at least one of the symptoms reported prior to treatment. None of the patients presented de novo facial palsy or deafness after GKR. Data on acute complications were obtained in 55 patients, 18.7% of the total. The reported acute complications were headache (23.6%), increased tinnitus (7.27%), nausea (5.45%), pain at fixation points (5.45%), increased vertigo (3.64%), facial paresthesias (1.82%), and temporary sensation of worsening hearing loss (1.82%). In 50.9%, there were no acute complications.
Metastases
187 procedures (9.81%) were performed, 64.7% on women and 35.3% on men. A total of 143 patients were treated, 29 of them were treated more than once due to the presence of new lesions. The mean coverage dosage was 19 Gy. The average age was 59 years (SD 13). Twelve patients (8.39%) had a history of whole-brain radiotherapy and one patient (0.7%) had a history of Linear Accelerator (LINAC) radiosurgery. In 22.3% of the patients, there was a history of previous cranial surgery. The most frequent primary origins were pulmonary (33.1%), breast (29.9%), melanoma (8.02%), renal (7.49%), and thyroid (7%). Other less frequent primary origins were prostate, colon, stomach, and liver, among others. In 3.7% of cases, the primary origin was unknown. Eight hundred and ninety-one lesions were treated in total. The median number of injuries per patient was 3 (IQR 1–6) with a maximum of 33. The median highest volume treated per fraction was 8.9 cc (IQR 4–15.6). About 64.5% of the procedures were performed in a single fraction and 35.5% were performed in two and three fractions. The median hospital stay was 5.67 h (IQR 3.7–26.2). The treatment data are shown in
At 1-year posttreatment, follow-up data were obtained from 40 patients, 27.9% of the total, with a median follow-up of 11.2 months (IQR 8.1–14.2). About 5% required additional whole-brain radiotherapy during the follow-up period. In 40% of the patients, the size of the lesions remained stable, in 47.5%, there was a decrease in size [
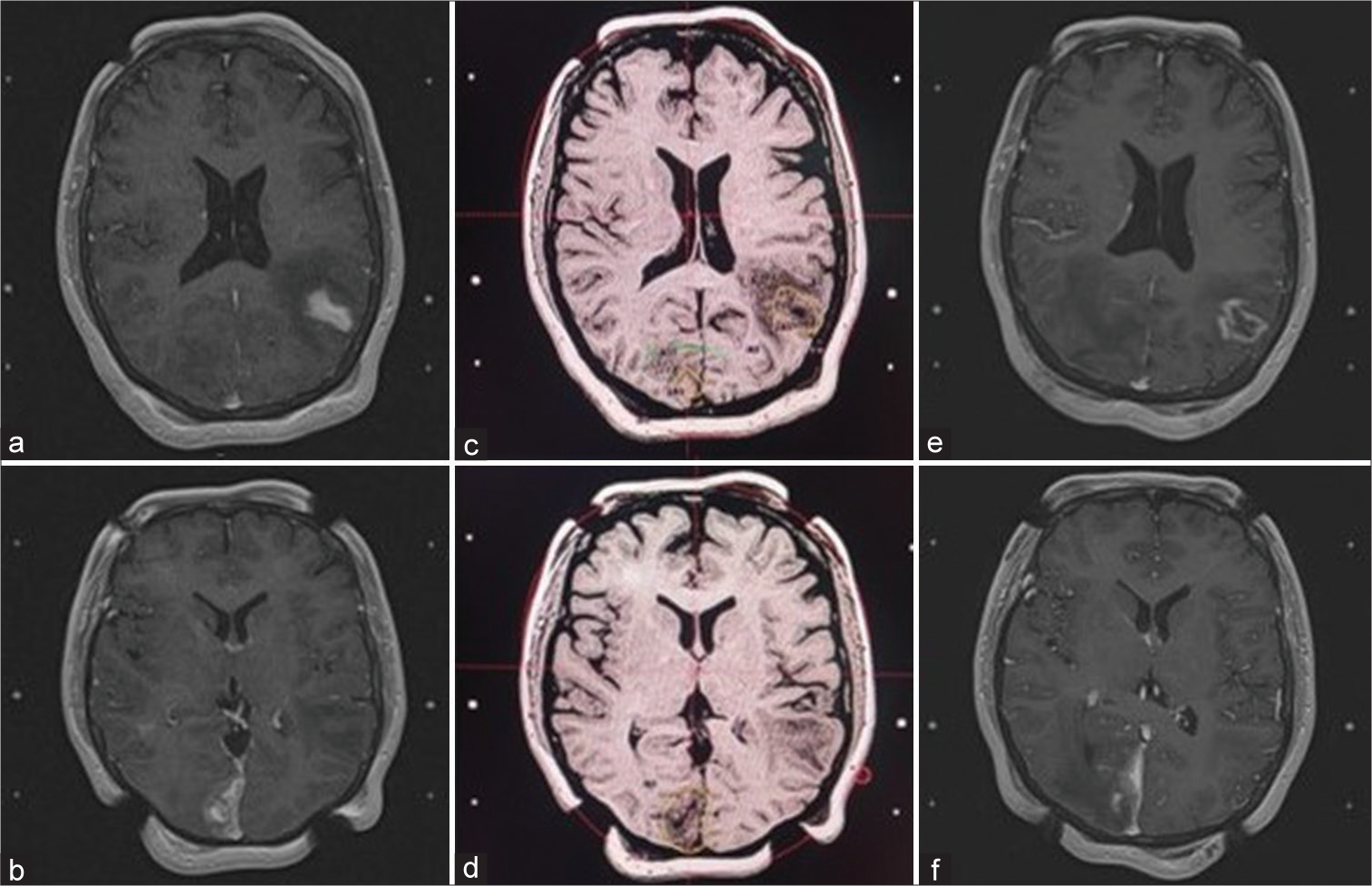
Figure 5:
A 39-year-old woman with a history of infiltrating ductal-type breast cancer with brain metastases. (a and b) Magnetic resonance imaging (MRI) shows a metastatic lesion in the left posterior temporal region with surrounding edema and another in the right paramedian occipital region. (c and d) MRI at the time of radiosurgery where it is observed the dose planning and isodose to the tumors. (e and f) MRI 8 months posttreatment, which was performed to plan a new treatment due to the presence of new lesions. It is observed a decrease in the size of the left temporal tumor, with radionecrosis changes inside and less edema around it. It is also evidenced a decrease in size of the right paramedian occipital tumor.
Trigeminal neuralgia and other facial pains
Including the group of other facial pains, a total of 181 procedures were performed, 65.7% on women and 34.3% on men. In total, 164 patients were treated. The mean maximum dose was 90 Gy. The median age was 64 years (IQR 53–73). Compromise of the right side of the face was described in 55.8% of the cases and of the left side in 44.2%. The most frequently affected branches were V2–V3 in 28.1%, only V3 in 16.5%, and V1–V2 in 14.3%. In 6% of patients, there was the involvement of the three branches. The primary origin was present in 81.7%, within which vascular conflict was described in 46.4%. On the other hand, a secondary cause was described in 18.2% of the patients, being the causes: tumor (39.3%), herpes zoster (33.3%), multiple sclerosis (18.1%), trauma (6%), and mastoiditis (3%).
About 18.2% of the patients had a history of previous surgery and 28.1% had a history of previous percutaneous procedures, of which Gasserian ganglion thermal radiofrequency was the most frequent followed by peripheral facial block and sphenopalatine ganglion block. Depending on the clinical characteristics, we decided to treat different targets. The most frequent was the retro-Gasserian portion of the trigeminal root (66.8%), generally in patients with no history of previous management. In 27% of cases, we included the centromedian nucleus of the contralateral thalamus as a complementary target, generally in cases with a history of multiple previous failed treatments and recurrent cases, as a physiopathological-based approach, which has the aim of interrupting nociceptive stimuli before arriving to the cerebral cortex in these complex cases. Other targets were also included, such as the sphenopalatine ganglion, in facial pain with an associated autonomic component; pontocerebellar angle tumors in some secondary facial pains; and bilateral anterior cingulotomy, which was performed on patients with refractory pain, emotional manifestations, and great compromise in their quality of life. For cingulotomy, two shots with a 4 mm collimator in each anterior third of the cingulum bundle are applied, with a maximum dosage of 120 Gy and monitoring doses under 30 Gy in the adjacent anterior cerebral arteries. All procedures in trigeminal neuralgia were performed in a single fraction. The median hospital stay was 3.98 h (IQR 2.8– 5.5). The treatment data are described in
At 1-year posttreatment, follow-up data were obtained from 95 patients, 57.9% of the total, with a median follow-up of 11.8 months (IQR 4.4–21.9). A decrease in pain intensity was described in 88.4% of the patients, among whom, in 64.3%, there was a decrease in the analgesic requirement. Recurrence of pain was described in 14 patients, corresponding to 16.6%, with an average time of recurrence of 55.3 months. Within the acute complications, data were obtained from 103 patients, 62.8% of the total. The described manifestations were headache (8.7%), nausea (4.8%), and pain at the frame fixation points (1.9%). In 89.3%, there were no acute complications. In the long term, the presence of paresthesias was described in 6.3% and there were no cases of painful numbness or corneal ulcers.
DISCUSSION
GKR is a multidisciplinary procedure that involves neurosurgery, neurointervention, neuroradiology, medical physics, and nursing services around the patient. To the best of our knowledge, this is the first detailed report on a large number of patients treated with GKR in Latin America. Our 12-year experience has enabled us to observe that it is a treatment with multiple benefits.
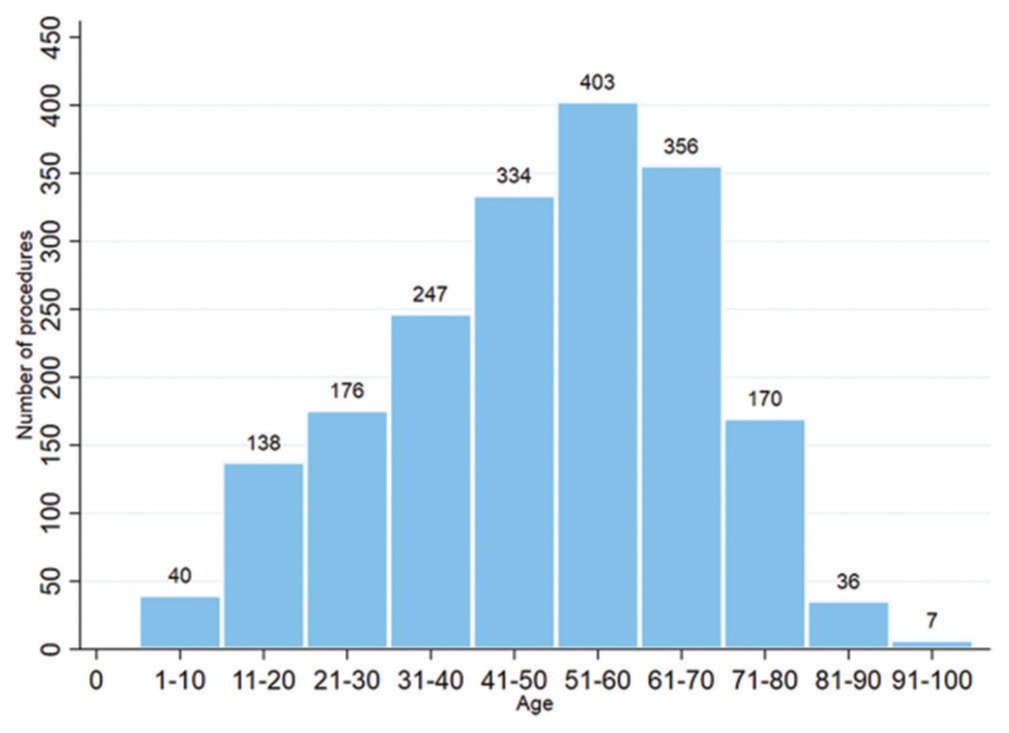
Patients with different pathologies and a wide range of ages have been treated, being patients between 41 and 70 years of age who receive the greatest number of treatments. It was related, in some cases, to one of the main indications for treatment, which is the presence of high perioperative risk or comorbidities such as obesity, chronic obstructive pulmonary disease (COPD), heart failure, and continuous use of anticoagulation, among others. The other three main indications were patients with tumoral and vascular lesions of difficult surgical approach, patients with a requirement of multiple targets treatment, and patients who voluntarily rejected the option of conventional surgical management.
GKR can be used in patients with a history of previous management, such as surgery, percutaneous management, or other radiation therapies. Depending on the needs of the patient, the procedure can be repeated and it has been seen that it generates lower costs than conventional surgery in our institution. The care process does not require surveillance in the intensive care unit, and also, the hospitalization stay is short. In addition, most procedures are outpatient and patients can return to their daily activities the next day, which decreases work disability. Postoperative pain and complications are generally mild and amenable to medical management.
During these years of experience, we have been able to treat more than 1 lesion simultaneously, which is especially useful in patients with metastasis, in whom we have treated up to 33 lesions in one session. In addition, as we show in
Along with the generalization in the use of this technique recently occurring in the world, the number of procedures in our institution has been increasing over the years, with a slight decrease in the number of procedures in 2020 and 2021, during the COVID-19 pandemic. However, it was a smaller decrease compared to the reduction presented in the number of conventional surgical procedures in our institution, which may be due to the noninvasive nature of GKR and less direct contact with the patient during the procedure. These characteristics, and personal protection measures, allowed us to maintain low contagion rates while continuing to respond to the needs of patients regarding their neurosurgical pathologies.
Regarding target localization, approximately 70% of the meningiomas treated by radiosurgery in our institution were located in the skull base and in the posterior fossa. Of the AVMs, 23.1% were located in the basal ganglia region and posterior fossa. Many of these lesions are not readily accessible, surgically speaking. In the case of schwannomas, we observed a high frequency of facial paralysis and complete hearing loss in patients with a history of previous surgery. This suggests that not only is GKR an alternative treatment to conventional surgical management, but, in selected cases, also it may be the best treatment option depending on the characteristics of the patient. In our experience, a low frequency of acute and long-term complications was described. In general, these complications were similar in the different diagnoses, except in vestibular schwannoma, in which a temporary worsening of tumor-related symptoms was observed in some cases. All acute complications were mild and transient.
Complications associated with frame placement have been reported in the literature, such as technical difficulty for placement, persistent pain, perforation, and infection.[
Compared with the experiences reported in high-income countries, specifically with the number of procedures per year,[
Limitations
This study was limited by the availability of information in clinical records, mainly in the follow-up data, which are not usually carried out in an optimal and timely manner due to administrative problems in the health system of our country. The main objective is to highlight the institutional experience in the management of patients with GKR. It is neither the objective of this review to make large causal associations nor compare the technique with other therapies. Additional studies are required to establish those comparisons.
CONCLUSION
Our experience suggests that GKR is a noninvasive procedure with a low frequency of complications, with good results in the medium and long term. It is feasible in terms of direct costs associated with the care process and costs derived from medical disabilities. Moreover, it is also a procedure with application to a broad spectrum of pathologies in a large range of ages, being an important option in patients with conventional surgical management contraindication.
Statement of ethics
This study protocol was reviewed and approved by the ethics committee of Fundación Clínica Shaio, Bogotá, Colombia. The approval number is CEI 089.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
To Dr. Fabio Echeverri Correa †, chair of the board, and to Dr. Gilberto Mejía, general director of the Clinica Shaio, because through their management, it was possible to have this technology in our institution, to put it at the service of our patients. To Dr. Paola Recaman, director of the education department, for providing us with the spaces for the development of this research. To Johana López, from the statistics department, for her invaluable support in managing the collected data.
References
1. Berkowitz O, Kondziolka D, Bissonette D, Niranjan A, Kano H, Lunsford D. The evolution of a clinical registry during 25 years of experience with Gamma Knife radiosurgery in Pittsburgh. Neurosurg Focus. 2010. 34: E4
2. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FK, Kornguth DG. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009. 10: 1037-44
3. Fujii O, Tsujino K, Soejima T, Yoden E, Ichimiya Y, Sugimura K. White matter changes on magnetic resonance imaging following whole-brain radiotherapy for brain metastases. Radiat Med. 2006. 24: 345-50
4. Larsson B, Leksell L, Rexed B, Sourander P, Mair W, Andersson B. The high-energy proton beam as a neurosurgical tool. Nature. 1958. 182: 1222-3
5. Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951. 102: 316-9
6. Lunsford LD, Flickinger J, Coffey RJ. Stereotactic Gamma Knife radiosurgery initial North American experience in 207 patients. Arch Neurol. 1990. 47: 169-75
7. Lunsford LD, Flickinger J, Lindner G, Maitz A. Stereotactic radiosurgery of the brain using the first United States 201 cobalt-60 source Gamma Knife. Neurosurgery. 1989. 24: 151-9
8. Monaco EA, Grandhi R, Niranjan A, Lunsford LD. The past, present and future of Gamma Knife radiosurgery for brain tumors: The Pittsburgh experience. Expert Rev Neurother. 2012. 12: 437-45
9. Pollock BE, Gorman DA, Schomberg PJ, Kline RW. The mayo clinic gamma knife experience: Indications and initial results. Mayo Clin Proc. 1999. 74: 5-13
10. Vulpe H, Save AV, Xu Y, Elliston CD, Garrett MD, Wu CC. Frameless stereotactic radiosurgery on the gamma knife icon: Early experience from 100 patients. Neurosurgery. 2020. 86: 509-16
11. Walton L, Bomford CK, Ramsden D. The Sheffield stereotactic radiosurgery unit: Physical characteristics and principles of operation. Br J Radiol. 1987. 60: 897-906