- Department or Neurosurgery and Radiosurgery, Fundación Clínica Shaio, Bogotá, Colombia
- Neurosurgery Program, Universidad El Bosque, Bogotá, Colombia
- Medicine Program, Universidad El Bosque, Bogotá, Colombia.
Correspondence Address:
Oscar I. Molina-Romero, Department of Neurosurgery and radiosurgery, Fundación Clínica Shaio, Neurosurgery Program, Universidad El Bosque, Bogotá Colombia.
DOI:10.25259/SNI_866_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Oscar I. Molina-Romero1,2, Andrés Fonnegra-Caballero1, Juan Carlos Diez-Palma1, Andrés Segura-Hernández1, Valentina Rodriguez-Noreña3, Gloria Segura-Hernández3, Valentina Corredor-Torres3, María Clara Rojas-Ortiz3, Diana Useche-Aroca3, Julio R. Fonnegra-Pardo1. Gamma Knife radiosurgery for the management of glomus jugulare tumors: A systematic review and report of the experience of a radioneurosurgery unit in Latin America. 08-Mar-2024;15:78
How to cite this URL: Oscar I. Molina-Romero1,2, Andrés Fonnegra-Caballero1, Juan Carlos Diez-Palma1, Andrés Segura-Hernández1, Valentina Rodriguez-Noreña3, Gloria Segura-Hernández3, Valentina Corredor-Torres3, María Clara Rojas-Ortiz3, Diana Useche-Aroca3, Julio R. Fonnegra-Pardo1. Gamma Knife radiosurgery for the management of glomus jugulare tumors: A systematic review and report of the experience of a radioneurosurgery unit in Latin America. 08-Mar-2024;15:78. Available from: https://surgicalneurologyint.com/surgicalint-articles/12788/
Abstract
Background: Glomus jugulare tumors (GJTs) are rare and mainly affect women between the 5th and 6th decades of life. Its localization and anatomic relationships make conventional surgical treatment difficult and with a considerable risk of complications. This manuscript aims to describe the results of Gamma Knife radiosurgery (GKR) in patients with GJT treated in a single center in Latin America, as well as to systematically review the literature to determine the clinical and radiological effectiveness of this technique.
Methods: A search of information from January 1995 to June 2023 was performed. Twenty-two articles reporting 721 GJT patients treated with GKR were included in the study. Variables such as symptomatic control, control of tumor size, and complications were evaluated. These variables were described using measures of central tendency and proportions. For the institutional experience, 77 patients with GJT tumors were included in the study. Pre-treatment clinical variables and follow-up data were collected from medical charts and phone interviews. The Short Form-36 scale was applied to assess the quality of life. The data were analyzed using the statistical program STATA17.0.
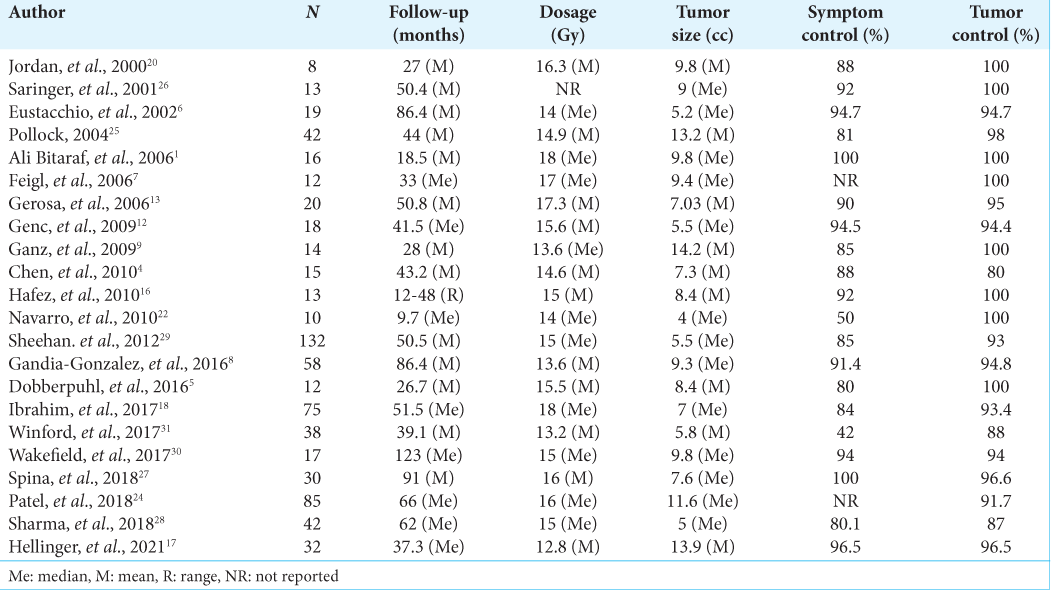
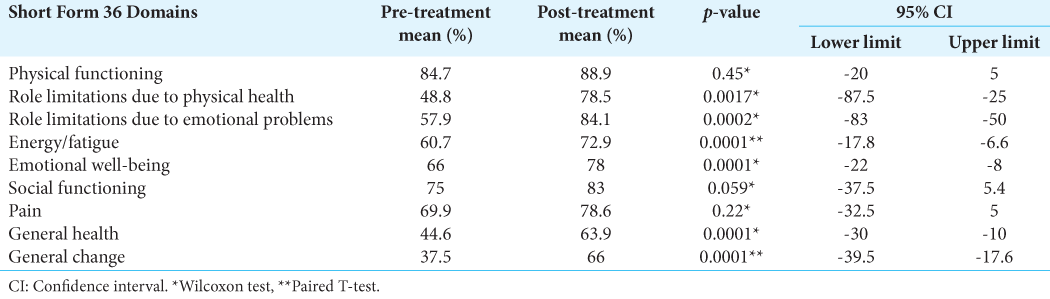
Results: A total of 721 patients were considered. The median of patients included in these studies was 18.5. The mean age was 58.4 years. The median of symptom control was 89%, and the median of imaging control was 95.7%. In our institution, 77 patients were included in the study. The mean age was 53.2 years. The median hospital stay was 4.92 hours. For the clinical follow-up, information on 47 patients was obtained. An improvement in pre-treatment symptoms was described in 58%, with general symptomatic control of 97%. The tumor-control rate was 95%, and there were statistically significant differences in six of the nine Short Form-36 scale domains.
Conclusion: GKR is an effective, safe, and cost-effective technique that offers a high degree of symptomatic and tumor size control in patients with GJT.
Keywords: Gamma Knife radiosurgery, Glomus jugulare, Head paraganglioma, Jugular paraganglioma, Stereotactic radiosurgery
INTRODUCTION
Glomus jugulare tumors (GJT), or paragangliomas, are rare tumors that arise from paraganglionic cells located in the adventitia of the jugular bulb in the jugular foramen.[
The clinical manifestations derived from its growth result in symptoms that are mostly annoying or disabling. The location of the jugular bulb at the base of the skull and its relationship with vascular and nervous structures makes conventional surgical treatment difficult and with a considerable risk of complications. It is necessary to study the effectiveness of non-invasive methods that achieve adequate symptomatic control with a minimum percentage of complications. This manuscript aims to describe the results of treatment with Gamma Knife radiosurgery (GKR) in patients with GJT treated in a single center in Latin America, as well as to systematically review the literature to determine the clinical and radiological effectiveness of this technique.
MATERIALS AND METHODS
The systematic review protocol was pre-specified and registered on PROSPERO (ID CRD42023441012), and it is based on the guidelines of the Preferred Reporting Items of Systematic Reviews and Meta-analysis (PRISMA).[
Search strategy
A search of information was conducted considering a period from January 1995 to June 2023 in MEDLINE, SCOPUS, and COCHRANE. We also included other references from the list of some studies of interest. To search for information, the following terms were used as keywords and terms included in the title and abstract: “Gamma Knife radiosurgery” OR “Stereotactic Radiosurgery” OR “GKRS” AND “Glomus tumor” OR “Glomus jugulare” OR “Jugular paraganglioma” OR “Glomus tympanicum” OR “Glomus jugulotympanicum” OR “Head paraganglioma” OR “Tympanic chemodectoma.”
Eligibility criteria
Studies were required to meet the following criteria to be eligible [ English language studies Studies performed in humans Clinical and/or imaging outcomes assessed and reported GKR as the selected stereotactic radiation technique
Two review authors (JRF, JCD) excluded clearly irrelevant titles and abstracts. Three review authors (OMR, AFC, ASH) independently reviewed full texts for eligibility based on the inclusion criteria. Disagreements were resolved through a discussion of the five reviewer authors.
Data and statistical analysis
Four review authors (OMR, AFC, ASH, JCD) independently extracted data into a data extraction form. This form was used to record the year of study, the number of patients treated, the dosage of radiation, tumoral size, length of follow-up, symptom control rate, imaging tumoral control rate, and complications.
Symptom control was defined as a decrease or no worsening of the symptoms reported before treatment. Imaging control was defined as the reduction or stabilization of the tumoral size measured with magnetic resonance imaging (MRI ). Two review authors (OMR, ASH), assessed the risk of bias qualitatively.
Report of institutional experience
We conducted a single-center retrospective observational study with the approval of the Institution’s Ethics Committee. Seventy-seven patients with GJT tumors were treated with Gamma Knife Perfexion between June 2010 and December 2022. Pre-treatment clinical variables and follow-up data were collected from medical charts and phone interviews. Follow-up data were obtained at two moments: (1) during the first month post-treatment to assess acute complications, and (2) between the 2nd and 3rd-year post-treatment to assess clinical and radiological response and chronic complications. Clinical response was defined as stability or improvement of symptoms reported before treatment. The Short Form-36 scale was applied to assess the quality of life, considering the prior and after-treatment state. The radiological response was defined as stability or decrease in tumor size assessed on brain MRI.
The data were analyzed using the statistical program STATA 17.0. For quantitative variables, normality in the distribution of the data was verified by graphs, the Shapiro–Wilk test, and kurtosis. According to the results, measures of central tendency were applied, mainly median with interquartile range (IQR) and mean with standard deviation (SD). In the case of qualitative variables, proportions were calculated. To compare the results of the Short Form-36 scale, paired t-tests, and the Wilcoxon test were used depending on the distribution of the data. P < 0.05 was considered as the cutoff to establish statistical significance.
RESULTS
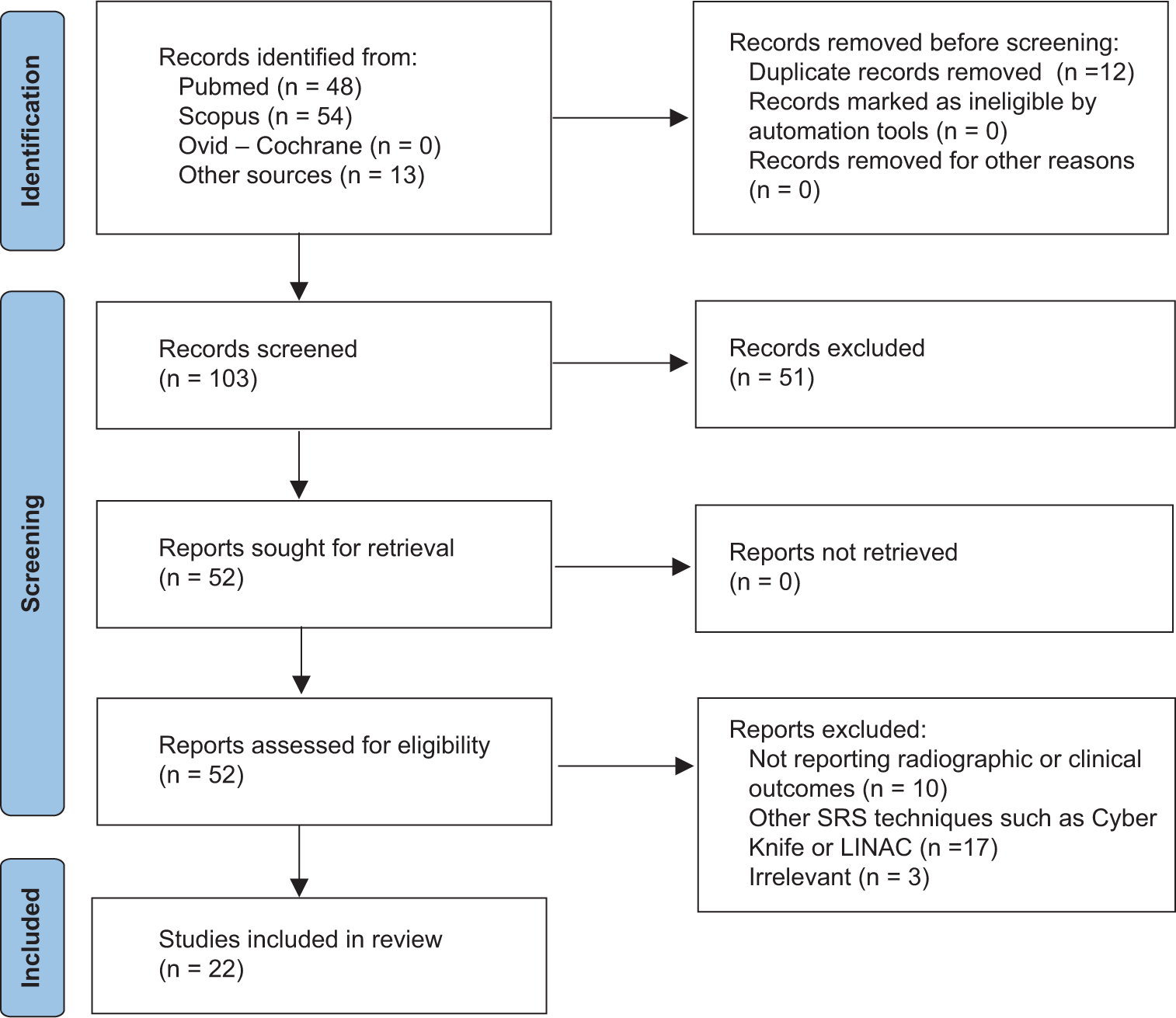
Twenty-two studies met the eligibility criteria and were included in the final qualitative analysis [
Regarding the complications reported, considering the total number of studies included, 18 cases of hearing impairment were reported;[
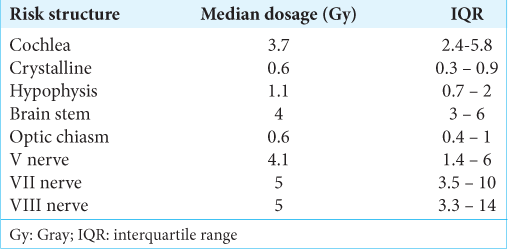
Of the 77 procedures performed with GKR, 72.7% were performed in a single fraction, 19.4% in two fractions, and 7.8% in three fractions. The median hospital stay was 4.92 h (IQR 3.4–23). Regarding treatment data, the median maximum dose used was 30 Gy (IQR 28–32), the median prescription dosage was 15 Gy (IQR 12.5–16), the median shots 26 (IQR 18 - 37), and the median volume of the lesions was 5.4 cc (IQR 3.38–13.2). The doses received by the structures at risk are described in
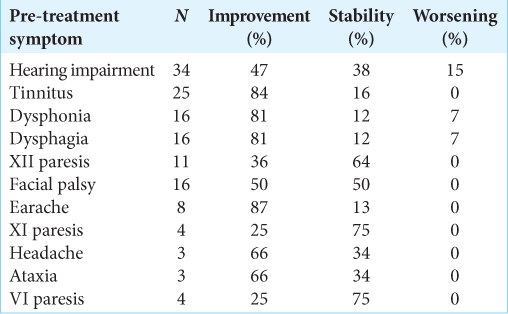
For the clinical follow-up, information on 47 patients was obtained, with a median follow-up of 46 months (IQR 23.3– 74.4). Of the symptoms reported before treatment with GKR, improvement was described in 58%, with general symptomatic control of 97% [
Data from 50 patients were obtained to evaluate acute complications during the 1st-month post-treatment. Transient headache occurred in 26%, nausea in 24%, and pain in fixation points of the frame in 10%. About 66% of the patients did not present any symptoms. Regarding chronic complications possibly attributed to treatment, data were obtained from 47 patients with a median of 46 months. Vertigo (6%), dysphagia (2.1%), dysphonia (2.1%), and transient facial paresthesia (2.1%) were described. No new cases of cophosis or facial paralysis attributable to radiation were described, and mortality was 0%.
DISCUSSION
The jugular bulb is a deep-located structure that goes through the jugular foramen. It has important anatomic relationships with structures such as the facial nerve (lateral), hypoglossal nerve (medial), vertebral artery (inferior), and auditory cavity (upper). In addition, it is closely related to the lower cranial nerves.
Conventionally, open surgery has been the cornerstone of the management of the GJT, with a reported tumor control rate of up to 92.1%.[
Adjunctive preoperative embolization has emerged as a complementary treatment in these patients with the aim of reducing the risk of intraoperative bleeding and achieving higher degrees of resection; however, it has been described that embolization alone produces a risk of ischemic events and permanent cranial neuropathy, due to the overlapping blood supply between these tumors and the cranial nerves (CNs).[
The data reported by the studies included in the present review suggest that GKR provides a high rate of symptomatic control in patients with GJT. These findings resemble those described in our series of patients, in whom improvement or stability of symptoms was achieved in 97%. In addition, a statistically significant improvement was found in most of the domains of the Short Form-36 scale, which indicates not only an isolated improvement in symptoms but also a positive impact on the quality of life, functionality, and general condition of these patients.
The tumoricidal effect of radiation generates control of tumor growth. This is essential within the therapeutic objectives since it has been observed that the growth of these tumors occurs not only at a macroscopic level but also has an infiltrative behavior toward small fascicles and the perineurium. This behavior generates reactive fibrosis and contributes to clinical manifestations.[
The caudal extension of some of these tumors has been reported as a possible limitation in the efficacy of the GKR in patients with GJT;[
In our series, the high percentage of complications in patients with a background of prior surgical management is striking, many of which were severe and permanent. The data found in the literature and from our experience show that in addition to the control of tumoral size and clinical manifestations, GKR is a treatment modality with a high profile of safety since the percentage of complications reported is usually low. These complications are mostly transitory and related to radiation-induced inflammation that tends to resolve with medical management. The mortality of the procedure is practically 0%.
We consider that in patients in whom radiosurgery is performed as primary management, the improvement in clinical parameters such as symptomatic control and quality of life could be even greater than the reported in our series, on account of we included patients with a background of surgical management in whom as aforementioned, complications are mostly permanent and severe.
In our experience, GKR has multiple advantages as the primary treatment of patients with GJT since it is an outpatient procedure with a short hospital stay, is noninvasive and does not require long hours of anesthesia or previous embolization; and its safety profile avoids permanent complications that require prolonged care and rehabilitation. All of the above reasons, in our opinion, make GKR a treatment that is not only effective and safe but also cost-effective in the treatment of these patients.
Limitations
The published studies correspond to observational studies, mostly case series, for which there is a considerable risk of bias. It must be taken into account when interpreting the internal and external validity of the results. On the other hand, for our institutional experience, there is a risk of recall bias in the comparison of pre-and post-treatment symptoms, mainly in the quality-of-life scale, which uses 36 parameters to measure the outcome.
CONCLUSION
GKR is an effective, safe, and cost-effective technique that offers a high degree of symptomatic and tumor size control in patients with GJT. Moreover, this modality of treatment can attain a significant improvement in the quality of life of these patients. Given its multiple benefits, this treatment modality could be considered the first line in the management of patients with GJT; nevertheless, more prospective studies or clinical trials are needed for a more accurate analysis of the results of this treatment.
Authors’ contributions
OIM: Conceive and write protocol design, data collection, data analysis, and paper redaction. AS, JCD, and AF: Data collection, data analysis, and data interpretation. JRF: Data collection, data analysis, data interpretation, and editorial supervision. VR, VC, MCR, GS, and DSU: Data collection and data analysis.
Ethics approval
This study protocol was reviewed and approved by the ethics committee of the institution (DIB 22-19).
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
The authors would like to thank Dr. Humberto Jimenez Mejía, chair of the board, and to Dr. Gilberto Mejía Estrada, General Director of Clinica Shaio, for their support and management of the advancement of technology in our institution. To Paola Mayorga and Uriel Chica, medical physicists from the radio neurosurgery unit, who work daily to provide the best care to our patients.
References
1. Bitaraf MA, Alikhani M, Tahsili-Fahadan P, MotieiLangroudi R, Zahiri A, Allahverdi M. Radiosurgery for glomus jugulare tumors: Experience treating 16 patients in Iran. J Neurosurg. 2006. 105: 168-74
2. Carlson ML, Driscoll CL, Garcia JJ, Janus JR, Link MJ. Surgical management of giant transdural glomus jugulare tumors with cerebellar and brainstem compression. J Neurol Surg B Skull Base. 2012. 73: 197-207
3. Cece JA, Lawson W, Biller HF, Eden AR, Parisier SC. Complications in the management of large glomus jugulare tumors. Laryngoscope. 1987. 97: 152-7
4. Chen P, Nguyen J, Payne S, Sheehan J, Hashisaki G. Treatment of glomus jugulare tumors with gamma knife radiosurgery. Laryngoscope. 2010. 120: 1856-62
5. Dobberpuhl MR, Maxwell S, Feddock J, St Clair W, Bush M. Treatment outcomes for single modality management of glomus jugulare tumors with stereotactic radiosurgery. Otol Neurotol. 2016. 37: 1406-10
6. Eustacchio S, Trummer M, Unger F, Schröttner O, Sutter B, Pendl G. The role of Gamma Knife Radiosurgery in the management of Glomus jugular tumours. Acta Neurochir Suppl. 2002. 84: 91-7
7. Feigl GC, Horstmann GA. Intracranial glomus jugulare tumors: Volume reduction with Gamma Knife surgery. J Neurosurg. 2006. 105: 161-7
8. Gandía-González ML, Kusak ME, Moreno NM, Sárraga JG, Rey G, Álvarez RM. Jugulotympanic paragangliomas treated with Gamma Knife radiosurgery: A single-center review of 58 cases. J Neurosurg. 2014. 121: 1158-65
9. Ganz J, Abdelkarim K. Glomus jugulare tumours: Certain clinical and radiological aspects observed following Gamma Knife radiosurgery. Acta Neurochir (Wien). 2009. 151: 423-6
10. Gartrell BC, Hansen MR, Gantz BJ, Gluth MB, Mowry SE, Aagaard-Kienitz BL. Facial and lower cranial neuropathies after preoperative embolization of jugular foramen lesions with ethylene vinyl alcohol. Otol Neurotol. 2012. 33: 1270-5
11. Gaynor BG, Elhammady MS, Jethanamest D, Angeli SI, Aziz-Sultan MA. Incidence of cranial nerve palsy after preoperative embolization of glomus jugulare tumors using Onyx. J Neurosurg. 2014. 120: 377-81
12. Genç A, Bicer A, Abacioglu U, Peker S, Pamir M, Kilic T. Gamma knife radiosurgery for the treatment of glomus jugulare tumors. J Neurooncol. 2009. 97: 101-8
13. Gerosa M, Visca A, Rizzo P, Foroni R, Nicolato A, Bricolo A. Glomus jugulare tumors: The option of gamma knife radiosurgery. Neurosurgery. 2006. 59: 561-8
14. Gottfried ON, Liu JK, Couldwell WT. Comparison of radiosurgery and conventional surgery for the treatment of glomus jugulare tumors. Neurosurg Focus. 2004. 17: E4
15. Guss ZD, Batra S, Li G, Chang SD, Parsa AT, Rigamonti D. Radiosurgery for glomus jugulare: History and recent progress. Neurosurg Focus. 2009. 27: E5
16. Hafez RF, Morgan MS, Fahmy OM. The safety and efficacy of gamma knife surgery in management of glomus jugulare tumor. World J Surg Oncol. 2010. 8: 76
17. Hellinger RL, Wolf A, Blach L, Kleinberg LR, Coy S. Long-term results of gamma knife radiosurgery for glomus tumors: An analysis of 32 patients. Cureus. 2021. 13: e18095
18. Ibrahim R, Ammori MB, Yianni J, Grainger A, Rowe J, Radatz M. Gamma Knife radiosurgery for glomus jugulare tumors: A single-center series of 75 cases. J Neurosurg. 2017. 126: 1488-97
19. Jackson CG, McGrew BM, Forest JA, Netterville JL, Hampf CF, Glasscock ME. Lateral skull base surgery for glomus tumors: Long-term control. Otol Neurotol. 2001. 22: 377-82
20. Jordan JA, Roland PS, McManus C, Weiner RL, Giller CA. Stereotactic radiosurgery for glomus jugulare tumors. Laryngoscope. 2000. 110: 35-8
21. Manolidis S, Shohet JA, Jackson CG, Glasscock ME. Malignant glomus tumors. Laryngoscope. 1999. 109: 30-4
22. Navarro Martín A, Maitz A, Grills IS, Bojrab D, Kartush J, Chen PY. Successful treatment of glomus jugulare tumours with gamma knife radiosurgery: Clinical and physical aspects of management and review of the literature. Clin Transl Oncol. 2010. 12: 55-62
23. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021. 372: n71
24. Patel NS, Carlson ML, Pollock BE, Driscoll CL, Neff BA, Foote RL. Long-term tumor control following stereotactic radiosurgery for jugular paraganglioma using 3D volumetric segmentation. J Neurosurg. 2019. 130: 379-87
25. Pollock BE. Stereotactic radiosurgery in patients with glomus jugulare tumors. Neurosurg Focus. 2004. 17: E10
26. Saringer W, Khayal H, Ertl A, Schoeggl A, Kitz K. Efficiency of Gamma knife radiosurgery in the treatment of glomus jugulare tumors. Minim Invasive Neurosurg. 2001. 44: 141-6
27. Spina A, Boari N, Gagliardi F, Bailo M, Del Vecchio A, Bolognesi A. Gamma Knife radiosurgery for glomus tumors: Long-term results in a series of 30 patients. Head Neck. 2018. 40: 2677-84
28. Sharma M, Meola A, Bellamkonda S, Jia X, Montgomery J, Chao ST. Long-term outcome following stereotactic radiosurgery for glomus jugulare tumors: A single institution experience of 20 years. Neurosurgery. 2018. 83: 1007-14
29. Sheehan JP, Tanaka S, Link MJ, Pollock BE, Kondziolka D, Mathieu D. Gamma Knife surgery for the management of glomus tumors: A multicenter study. J Neurosurg. 2012. 117: 246-54
30. Wakefield DV, Venable GT, VanderWalde NA, Michael LM, Sorenson JM, Robertson JH. Comparative neurologic outcomes of salvage and definitive gamma knife radiosurgery for glomus jugulare: A 20-year experience. J Neurol Surg Part B Skull Base. 2017. 78: 251-5
31. Winford TW, Dorton LH, Browne JD, Chan MD, Tatter SB, Oliver ER. Stereotactic radiosurgical treatment of glomus jugulare tumors. Otol Neurotol. 2017. 38: 555-62