- Department of Medical Education, School of Medicine, Marian University College of Osteopathic Medicine, Indianapolis, United States.
- Department of Psychological and Brain Sciences, Indiana University, Bloomington, Indiana, United States.
- Department of Neurological Surgery, Oregon Health and Science University, Portland, Oregon, United States.
- Department of Pediatrics, Section of Pediatric Infectious Disease, Riley Hospital for Children, United States.
- Department of Neurological Surgery, Section of Pediatric Neurosurgery, Riley Hospital for Children, Indiana University School of Medicine, Indianapolis, Indiana, United States.
Correspondence Address:
Jeffrey S. Raskin, Department of Neurological Surgery, Section of Pediatric Neurosurgery, Riley Hospital for Children, Indiana University School of Medicine, Indianapolis, Indiana, United States.
DOI:10.25259/SNI_164_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nicole H. Chicoine1, Jackson Griffith-Linsley2, Joling Goh3, John J. Manaloor4, Jeffrey S. Raskin5. Giant Actinomyces brain abscess in an immunocompetent child: A management strategy. 06-Jul-2021;12:325
How to cite this URL: Nicole H. Chicoine1, Jackson Griffith-Linsley2, Joling Goh3, John J. Manaloor4, Jeffrey S. Raskin5. Giant Actinomyces brain abscess in an immunocompetent child: A management strategy. 06-Jul-2021;12:325. Available from: https://surgicalneurologyint.com/surgicalint-articles/10953/
Abstract
Background: Intraparenchymal brain abscess is a collection of microbes caused by inoculation through direct extension or hematogenous spread. Although rare, intraparenchymal abscesses are potentially fatal and can be detected when patients are symptomatic due to local mass effect on adjacent neural tissue. Brain abscess treatment includes medical management with appropriate antibiotics alone or medical management in combination with surgical debridement. Treatment strategies depend on the size and location of disease, as well as the virulence of the microorganism. Similar to medical management strategies, surgical strategies among providers are not uniform, with variation in approaches from complete extirpation of the abscess, including the abscess wall, to minimally invasive stereotactic needle aspiration. In particular, for children, there are no guidelines for therapy.
Case Description: We report a case of giant Actinomycosis right frontal brain abscess in an immunocompetent child without risk factors. A review of the literature for the treatment of brain abscess caused very rarely by Actinomyces in children is performed.
Conclusion: Successful treatment of brain access depends on organism and location. The even more uncommon giant intraparenchymal abscesses can be managed with minimal access and prolonged antibiosis, especially when slow-growing organisms are identified. Long-term follow-up should be employed to mitigate missed late failures.
Keywords: Actinomyces, Brain abscess, Medical management, Surgical
INTRODUCTION
Intraparenchymal brain abscesses have been recognized since the early days of modern medicine, with the first recorded treatment of a brain abscess in 1768 by French Surgeon Monrand.[
Diagnostic evaluation for possible brain abscess is critical and should include a medical workup with complete blood count, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR). Blood cultures should be obtained in febrile patients, especially those with concern for concomitant extra-axial infection.[
Management of a brain abscess utilizes a multifocal approach. The medical antibiotic therapy can be guided by operatively collected positive cultures with susceptibility testing of recovered microbes, to deescalate broad empiric pharmacotherapy. When one or more organisms are identified and developed abscesses are <2.5 cm in diameter, they are commonly treated with antibiotics alone, guided by a hypothesis that the minimum inhibitory concentration at abscess center is sufficiently toxic to the microorganism.[
There is a high degree of variability in both the antibiotic and surgical strategies for brain abscesses, but overall there are some commonalities, as follows: [
Treatment is often prolonged, lasting a minimum of 6 weeks Selected antibiotics should possess bactericidal properties Selected antibiotics must be able to cross the blood– brain barrier In nonoperative or culture-negative cases, the antimicrobial spectrum of activity should cover, at minimum, common Gram-positive and anaerobic organisms.
We present a 2-year-old female patient who presented to our practice with a giant right frontal Actinomyces abscess. This presentation of Actinomycosis is highly unusual as there were no identified risk factors, and development of an abscess without risk factors is not frequently reported in literature.
Due to the giant size of the abscess, it required multiple surgical strategies and interventions, ultimately resolving successfully without neurologic complication. The variability in operative approach further demonstrates the immense variability required in management of even one patient, to have resolution of symptoms, and the unusual nature of this case.
CASE REPORT
History
Our 2-year-old patient is the product of a 40-week gestation born through induced vaginal delivery to a Group B Streptococcus agalactiae negative mother. She developed normally, without infections, until age 8 months when she developed a febrile urinary tract infection from Escherichia coli that was managed with daily prophylactic trimethoprimsulfamethoxazole for 1 year without recurrence of UTI.
Examination
The patient presented to our emergency department at the age of 2 years 10 months with 3 weeks of headaches, lethargy, malaise, and following 2 days of vomiting before admission. She was afebrile without chills, rashes, recent infections, or on the infectious disease team’s evaluation without dental carries. She had only recent domestic travel, without international trips or exposures.
Her vital signs on presentation were normal and her clinical examination reflected an awake, alert but low-energy female with the left lower facial droop. Urine culture on presentation falsely recovered >100,000 colony-forming units/mL of E. coli, as a repeat culture before starting antibiotics returned negative. Her total white blood cell count was elevated at 15,000, with a neutrophil predominance (66%). She had an elevated ESR of 53 mm/h, as well as CRP which was increased at 6.3 mg/dl. Her preantibiotic therapy admission blood cultures did not recover E. coli and were finalized as negative. The symptom of facial droop prompted brain magnetic resonance imaging (MRI) [
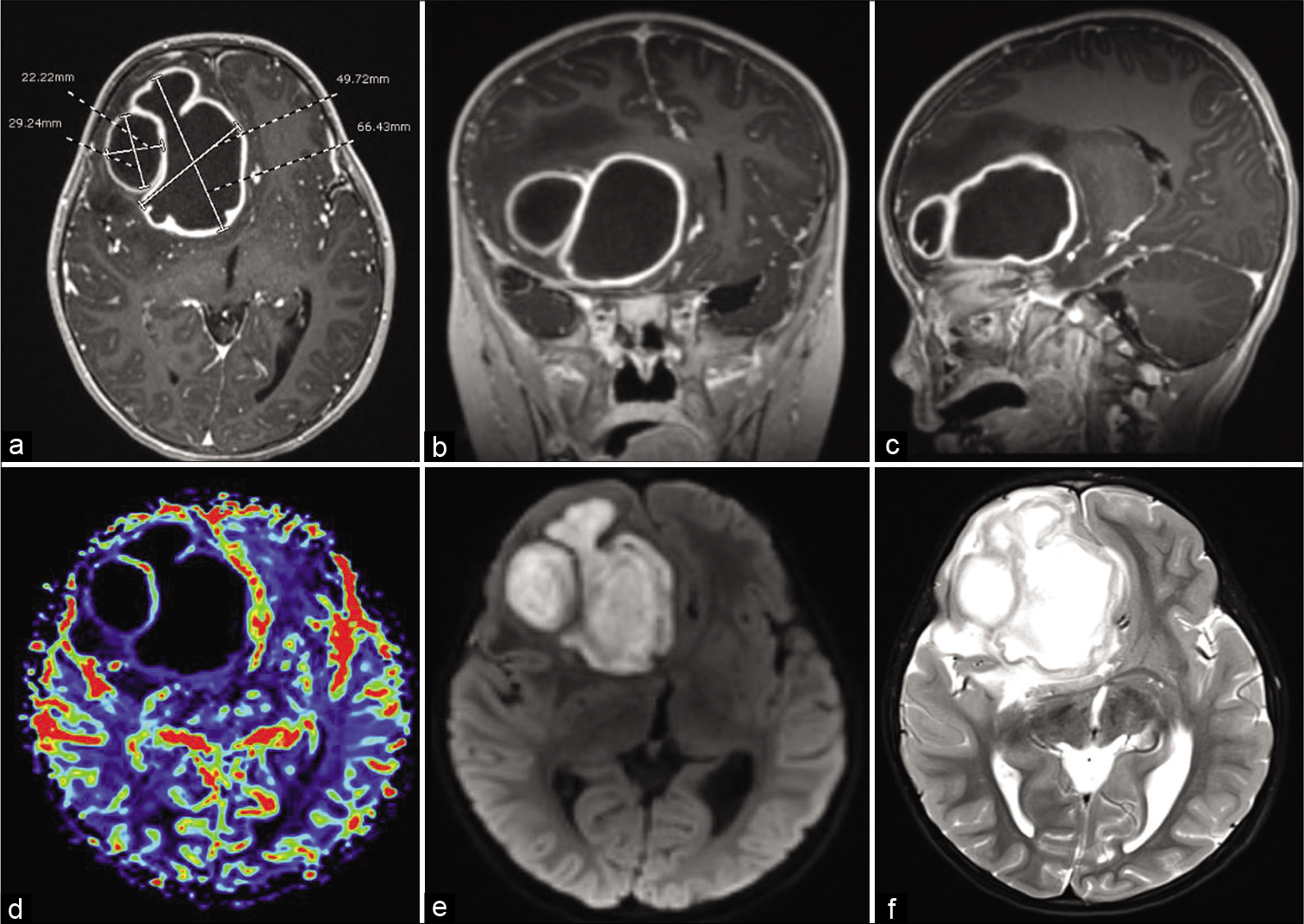
Figure 1:
Preoperative (a) axial, (b) coronal, (c) parasagittal T1 postgadolinium demonstrates two large ring-enhancing right frontal lesions causing significant mass effect, subfalcine herniation, and 19 mm midline shift. (d) Axial cerebral blood flow study demonstrates no blood flow within lesions, (e) axial diffusion restriction sequence, (f) axial T2 demonstrates extent of cerebral edema.
Operation
Consequently, the child underwent right frontal burr hole craniectomy and ultrasound-guided needle aspiration with removal of 50 mL of purulent material, a with a Gram stain detecting Gram-positive cocci. Initially, the patient was empirically treated with broad-spectrum intravenous (IV) triple antimicrobial therapy using vancomycin, cefepime, and metronidazole. Promptly, Actinomyces grew in the operative anaerobe culture within 48 h and was found to be pansusceptible. Subsequently, a peripherally inserted central catheter (PICC) was inserted and the child was converted to a continuous IV penicillin G (6,000,000 units/day), infused over 22 h, with oral metronidazole 120 mg every 6 h.
Postoperative course
The patient was discharged home on postoperative day (POD) 6 with a 2-week Decadron taper given her cerebral edema and prophylactic levetiracetam therapy. Eleven days later, she returned to the emergency department with a PICC-associated nonocclusive thrombus with a white blood cell elevation to 19,000, but an ESR of 5 (peak 60) and CRP of <0.5 (peak 6.3). Her PICC line was replaced in the opposite arm.
Seventeen days postoperatively, she represented to the emergency department with fever, emesis, and return of the left lower facial droop and continued headaches. Despite compliance with the prescribed antibiotic therapy, and in the absence of symptoms consistent with an alternative localizing infection, the white blood cell was found to be 11,800, with interval increases in ESR (70) and CRP (3.6). MRI, at this time, demonstrated a larger abscess in all dimensions. Due to radiographic worsening, recurrent symptoms, and worse inflammatory markers, the child was taken back to the operating room for aspiration and placement of an intra-abscess drain, and briefly provided IV vancomycin, changing penicillin to ceftriaxone. [
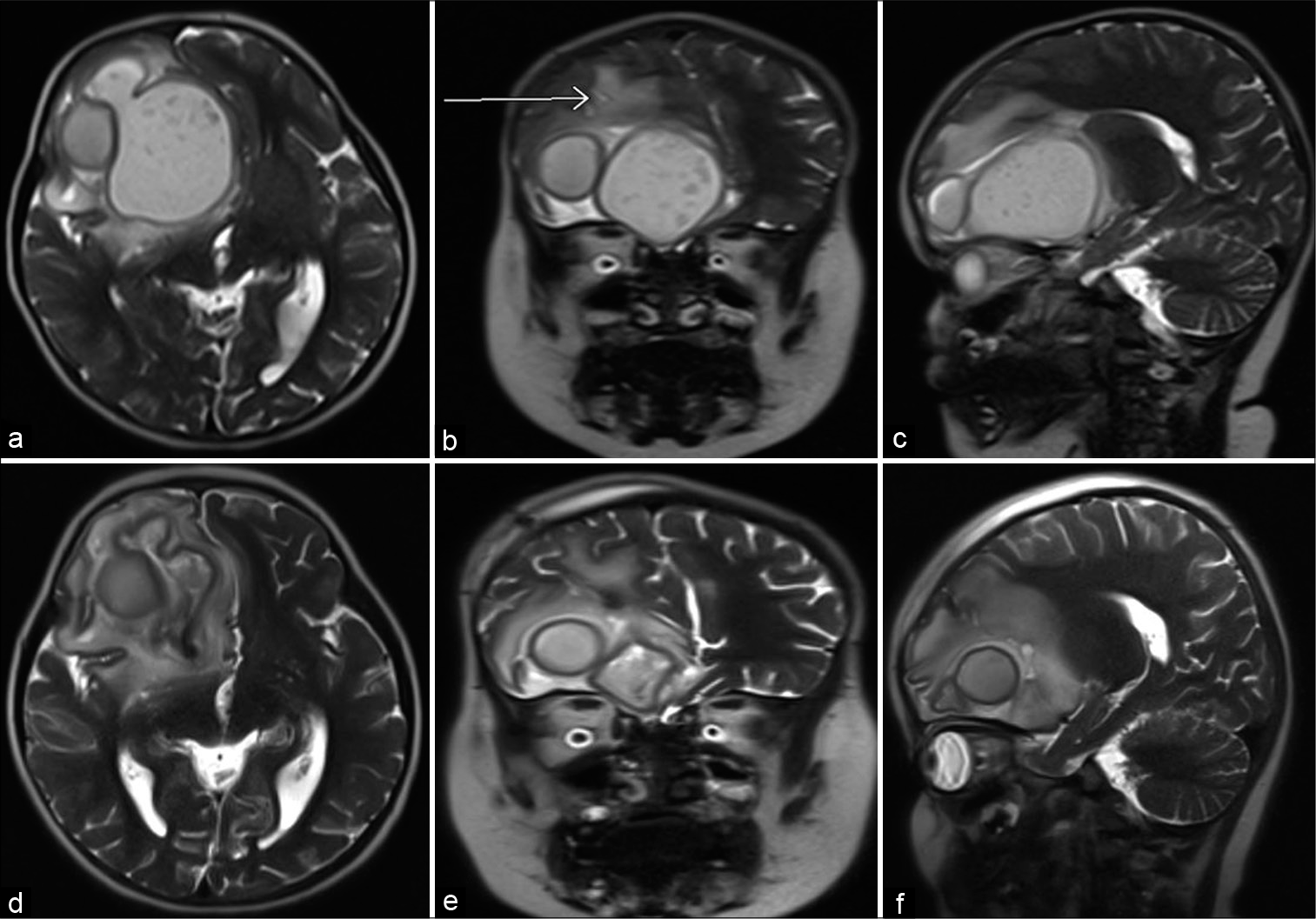
Figure 2:
Preoperative (a) axial, (b) coronal, (c) parasagittal T2 demonstrates slightly larger brain abscesses compared with previous imaging, stable mass effect, arrow points to previous needle tract. Postoperative (d) axial, (e) coronal, (f) parasagittal T2 demonstrates decompression of giant frontal abscess, stable size of the loculated satellite abscess measuring 2 cm.
The drain had daily decreased output from 30 mL, 19 mL, 11 mL, 8 mL, 5.5 mL, 3 mL, 2 mL, and 1.5 mL and was discontinued on POD 9. Antibiotic therapy was limited to ceftriaxone and oral metronidazole and since there was no growth of organism from any operative specimen collected during this third admission.
After 13 weeks of ceftriaxone and metronidazole following her second, and definitive, surgery, she is neurologically normal without developmental delay. [
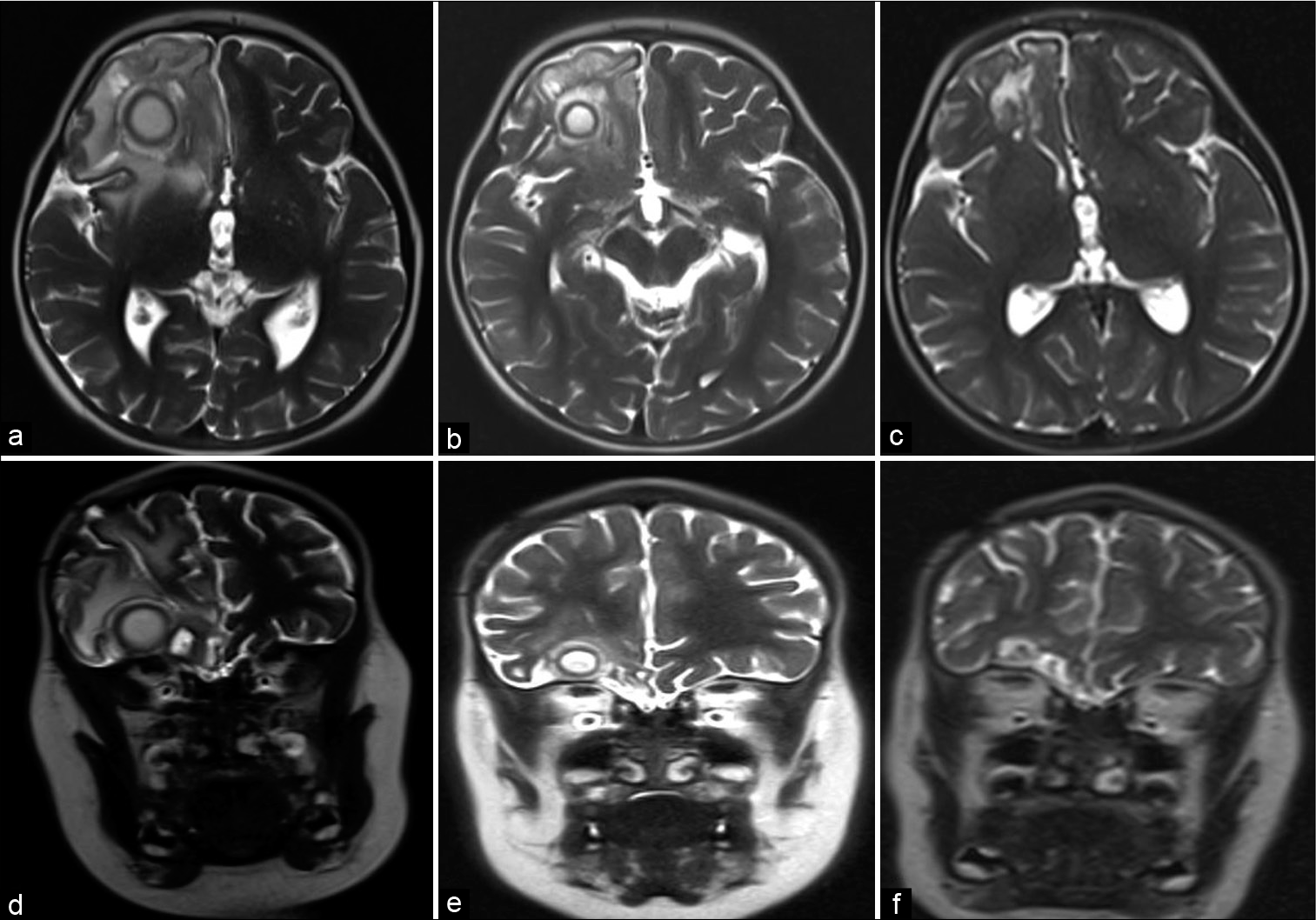
Figure 3:
T2 MRI following aspiration and drain placement of the right frontal giant abscess at discharge (a) axial and (b) coronal, at 6 weeks follow-up (c) axial and (d) coronal, and at 3 months follow-up (e) axial and (f) coronal. There is progressive improvement in cerebral edema, mass effect, and ultimately obliteration of the abscesses.
One month following treatment cessation, she developed fever and abdominal pain with transaminitis not previously seen during antimicrobial therapy. She was diagnosed with cholelithiasis, to which her prolonged ceftriaxone therapy likely contributed, and underwent an uncomplicated cholecystectomy. Surgical pathology demonstrated a gallbladder with some irregular cholelithiasis but was otherwise normal. The patient is currently in preschool and learning to be potty trained.
DISCUSSION
Otto Bollinger first discovered Actinomyces in 1877 in cattle, with subsequent recovery of the bacteria in soil by Eugen Bosteon.[
The low rate of confirmed Actinomyces diagnosis is likely confounded by the difficulty of recovering this microbe in the laboratory, on account of slow growth and strict anaerobic metabolism. Growth on the enriched medium of chocolate blood agar can take anywhere from 5 to 20 days and cannot be reasonably excluded without a minimum 10 days of “no growth” to be considered negative.[
Typically, cerebral abscesses from Actinomyces affect adults with predisposing factors including congenital heart defects, chronic otitis media, otologic surgery, chronic sinus infections, dental infections, alcoholism, IV drug use, and infected intrauterine device.[
On recognition of these abscesses, the management of CNS Actinomyces infection is multimodal. Despite the acquisition of susceptibility data, the antibiotic regimen for Actinomyces specific has immense variability. [
CNS Actinomyces infections are found in patients of all ages, although more cases reported in literature, are in the adult population. Within the pediatric population, there are a limited number of reported cases, and exceedingly rare, are those in an immunocompetent child without notable risk factors. We found no such cases in our literature review of an immunocompetent pediatric patient, like ours, developing an Actinomyces brain abscess. The common tie between pediatric and adult cases is the variability in the management of both antimicrobial and surgical approaches. In the case of a 2-year-old patient who uniquely had no risk factors, we found success utilizing a minimal access surgery with placement of intra-abscess drain and 3 months of antibiosis using ceftriaxone with metronidazole. We recommend this management in the future for pediatric patients with CNS Actinomyces abscesses.
CONCLUSION
Brain abscess is a rare consequence of hematogenous spread or direct extension of adjacent infection, and without appropriate often combinatorial treatment can frequently prove fatal. Successful treatment depends on organism and location. The even more uncommon giant intraparenchymal abscesses can be managed with minimal access and prolonged antibiosis, especially when slow-growing organisms are identified. Long-term follow-up should be employed to mitigate missed late failures.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Akhaddar A, Elouennass M, Baallal H, Boucetta M. Focal intracranial infections due to Actinomyces species in immunocompetent patients: Diagnostic and therapeutic challenges. World Neurosurg. 2010. 74: 346-50
2. Alvis MH, Castellar-Leones SM, Elzain MA, MoscoteSalazar LR. Brain abscess: Current management. J Neurosci Rural Pract. 2013. 4: S67-81
3. Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001. 48: 5-16
4. Arlotti M, Grossi P, Pea F, Tomei G, Vullo V, de Rosa FG. Consensus document on controversial issues for the treatment of infections of the central nervous system: Bacterial brain abscesses. Int J Infect Dis. 2010. 14: S79-92
5. Brook I. Aerobic and anaerobic bacteriology of intracranial abscesses. Pediatric Neurol. 1992. 8: 210-4
6. Chen M, Low SY, Muzumdar D. Management of brain abscesses: Where are we now?. Childs Nerv Syst. 2018. 34: 1871-80
7. Clancy U, Ronayne A, Prentice MB, Jackson A. Actinomyces meyeri brain abscess following dental extraction. BMJ Case Rep. 2015. 13: 2015
8. Corcione S, Curtoni A, Paolucci IA, di Perri G, de Rosa FG, Cavallo R. Neurological disease may precede lymphadenopathies in Actinomyces Europaeus infection. J Infect Public Health. 2018. 11: 592-3
9. Greenberg MS.editors. Handbook of Neurosurgery. New York: Thieme; 2016. p. 320-6
10. Guillamet LJ, Malinis MF, Meyer JP. Emerging role of Actinomyces meyeri in brain abscesses: A case report and literature review. IDCases. 2017. 10: 26-9
11. Hall A, White MA, Gallo P. An intra-cerebral abscess in a patient with Eisenmenger syndrome: An unusual case. Int J Surg Case Rep. 2016. 20: 138-41
12. Heineman HS, Braude AI. Anaerobic infection of the brain. Observations on eighteen consecutive cases of brain abscess. Am J Med. 1963. 35: 682-97
13. Hwang CS, Lee H, Hong MP, Kim JH, Kim KS. Brain abscess caused by chronic invasive actinomycosis in the nasopharynx: A case report and literature review. Medicine (Baltimore). 2018. 97: e0406
14. Jang Y, Moon J, Jun JS, Kim TJ, Park KI, Lee ST. Case of Rickettsia typhiinduced brain abscess mimicking brain tumor. Osong Public Health Res Perspect. 2018. 9: 122-5
15. Moal G, Landron C, Grollier G, Bataille B, Roblot F, Nassans P. Characteristics of brain abscess with isolation of anaerobic bacteria. Scand J Infect Dis. 2003. 35: 318-21
16. Muzumdar D, Jhawar S, Goel A. Brain abscess: An overview. Int J Surg. 2011. 9: 136-44
17. Muzumdar D. Central nervous system infections and the neurosurgeon: A perspective. Int J Surg. 2011. 9: 113-6
18. Mylonas A, Tzerbos FH, Mihalaki M, Rologis D, Boutsikakis I. Cerebral abscess of odontogenic origin. J Craniomaxillofac Surg. 2007. 35: 63-7
19. Ravindra N, Sadashiva N, Mahadevan A, Bhat DI, Saini J. Central nervous system actinomycosis-a clinicoradiologic and histopathologic analysis. World Neurosurg. 2018. 116: e362-70
20. Savardekar AR, Krishna R, Arivazhagan A. Spontaneous intraventricular rupture of pyogenic brain abscess: A short series of three cases and review of literature. Surg Neurol Int. 2016. 7: S947-51
21. Sharma RR, Apollina S. Cranio-cerebral abscesses in nocardiosis and Actinomycosis: Assessment and management strategies. JSM Brain Sci. 2017. 2: 6
22. Sheehan JP, Jane JA, Ray DK, Goodkin HP. Brain abscess in children. Neurosurg Focus. 2008. 24: E6
23. Shiordia J, Lopez-Mariscal C, Reynoso-Gonzalez R, Manriquez-Mejia M. Abdominal Actinomycosis: Case report and literature review. Acad J Microbiol Res. 2016. 4: 15-26
24. Stephanov S. Surgical treatment of brain abscess. Neurosurgery. 1988. 22: 724-30
25. Stone JL. Paul Broca and the first craniotomy based on cerebral localization. J Neurosurg. 1991. 75: 154-9
26. Valour F, Senechal A, Dupieux C, Karsenty J, Lustig S, Breton P. Actinomycosis: Etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014. 7: 183-97
27. Vidanaral AH, Kasper K, Karlowsky J, Walkty A. Successful treatment of Bacillus cereus group brain abscesses with a combination of high-dose ciprofloxacin and vancomycin. Official J Assoc Med Microbiol Infect Dis Can. 2017. 2: 64-8
28. Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ. 2011. 343: 785-90