- Department of Neurological Surgery, School of Medicine, Wakayama Medical University, Wakayama City, Japan.
Correspondence Address:
Kentaro Mineji, Department of Neurological Surgery, School of Medicine, Wakayama Medical University, Wakayama City, Japan.
DOI:10.25259/SNI_529_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kentaro Mineji, Rie Yako, Naotsugu Toki, Masaki Tomobuchi, Naoyuki Nakao. Giant cell arteritis with severe intracranial involvement diagnosed and treated early. 15-Sep-2023;14:332
How to cite this URL: Kentaro Mineji, Rie Yako, Naotsugu Toki, Masaki Tomobuchi, Naoyuki Nakao. Giant cell arteritis with severe intracranial involvement diagnosed and treated early. 15-Sep-2023;14:332. Available from: https://surgicalneurologyint.com/surgicalint-articles/12555/
Abstract
Background: Ischemic cerebrovascular accidents (CVA) occur in 3.3–7.2% of patients with giant cell arteritis (GCA), and intracranial vessels are rarely affected. We, herein, report a case of intracranial GCA with rapidly progressive multiple intracranial vascular lesions.
Case Description: A 76-year-old woman visited a local doctor due to a headache; then, it improved spontaneously. Three months later, she suddenly had cerebral infarctions of bilateral pons and cerebellum. Magnetic resonance angiography (MRA) revealed the left internal carotid artery (ICA) occlusion, the right vertebral artery (VA) occlusion, and the left VA stenosis. She was diagnosed with atherothrombotic stroke and dual antiplatelet therapy was administered. However, 2 weeks later, the left VA stenosis was aggravated. Therefore, we reviewed the data of MRA performed 3 months ago and noted no lesions in the ICA and VA. T1 black-blood post-gadolinium imaging sequence magnetic resonance imaging (MRI) revealed vessel wall enhancement in the bilateral VA, left ICA, and bilateral superficial temporal artery. We performed a temporal artery biopsy and diagnosed her with GCA. The progression of the intracranial vascular lesions was decelerated by oral glucocorticoid administration.
Conclusion: Intracranial vascular lesions in GCA can be formed later than initial symptoms, such as headache, and aggravated despite improvement in headache. In patients with GCA, evaluating intracranial vessels as a control is useful for distinguishing them from arteriosclerotic lesions at the onset of CVA. Intracranial GCA is characterized by rapidly progressive vascular lesions in the bilateral ICA and VA. In addition, T1 black-blood post-gadolinium imaging sequence MRI may lead to early diagnosis and treatment.
Keywords: Giant cell arteritis, Ischemic cerebrovascular accidents, Multiple intracranial vascular lesions, Rapidly progressive lesions
INTRODUCTION
Giant cell arteritis (GCA) is a primary large-vessel vasculitis found commonly in older people aged 50 or more years and mainly affects the extracranial branches of the carotid artery.[
CASE DESCRIPTION
A 76-year-old woman visited a local doctor immediately after she experienced headaches in the left and right temporal regions of the head. Magnetic resonance imaging (MRI) results showed no remarkable abnormal findings, and the doctor took a wait-and-see approach. The headache resolved spontaneously and did not relapse afterward. The patient had a history of hypertension and was undergoing treatment with oral antihypertensives.
Three months later, the patient visited our hospital because she suddenly experienced slurred speech and lightheadedness. Physical findings included disorientation, dysarthria, ataxic symptoms in the right upper and lower limbs, and inability to walk. MRI revealed infarction lesions in the left and right sides of the pons and cerebellum [
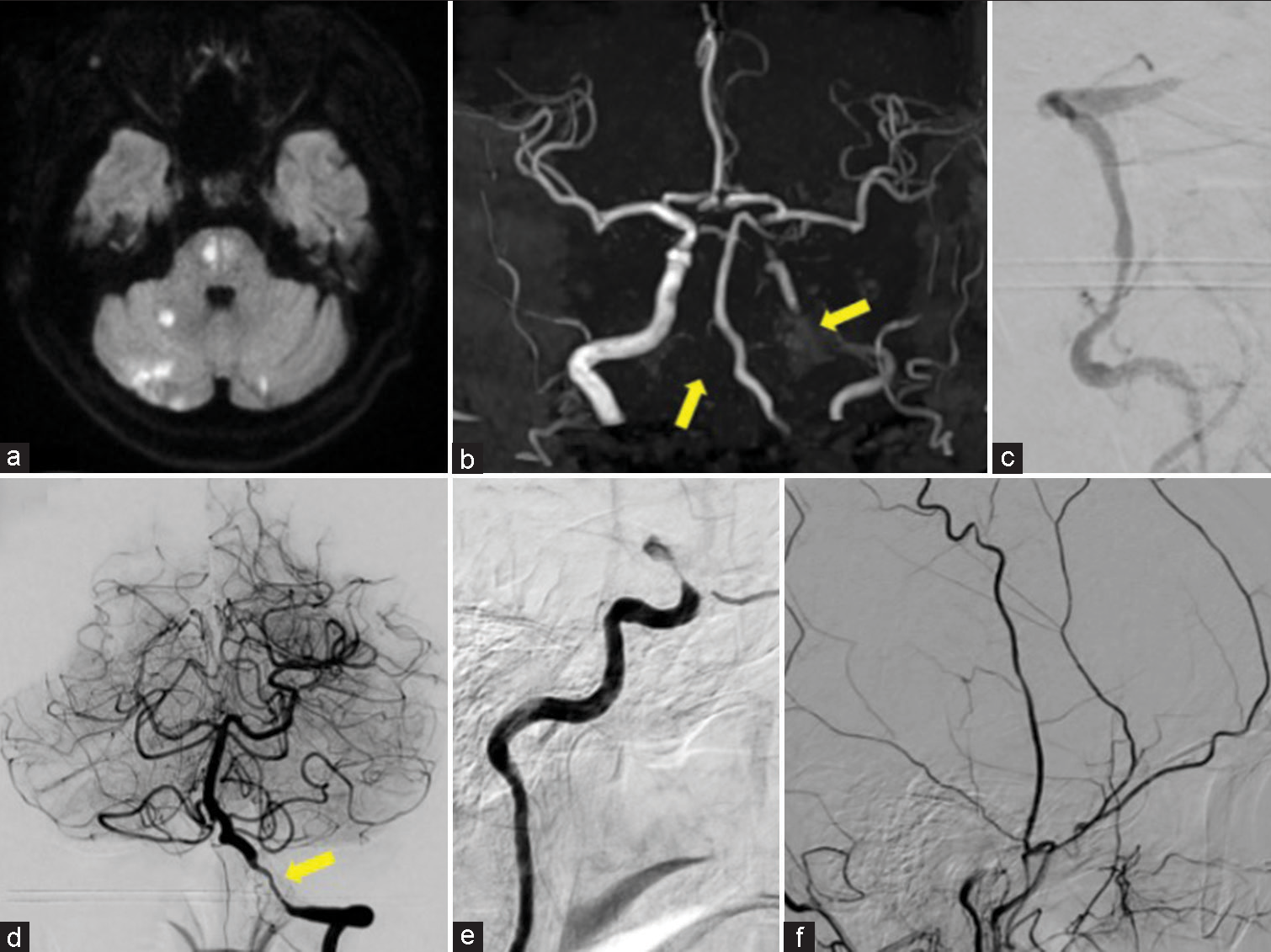
Figure 1:
(a) Diffusion-weighted image revealed cerebral infarctions in the left and right sides of the pons and cerebellum, and (b) the left internal carotid artery (ICA) and the right vertebral artery (VA) were not well visualized in magnetic resonance angiography (arrow). Digital subtraction angiography revealed (c) occlusion of the V4 portion of the right VA and (d) stenosis of the V4 portion of the left VA (arrow), as well as (e) occlusion of the left supraclinoid ICA. (f) Meanwhile, no clear stenosis of the superficial temporal artery was observed.
However, dysarthria was aggravated 2 weeks later. MRI revealed a new cerebral infarction in the left pons [
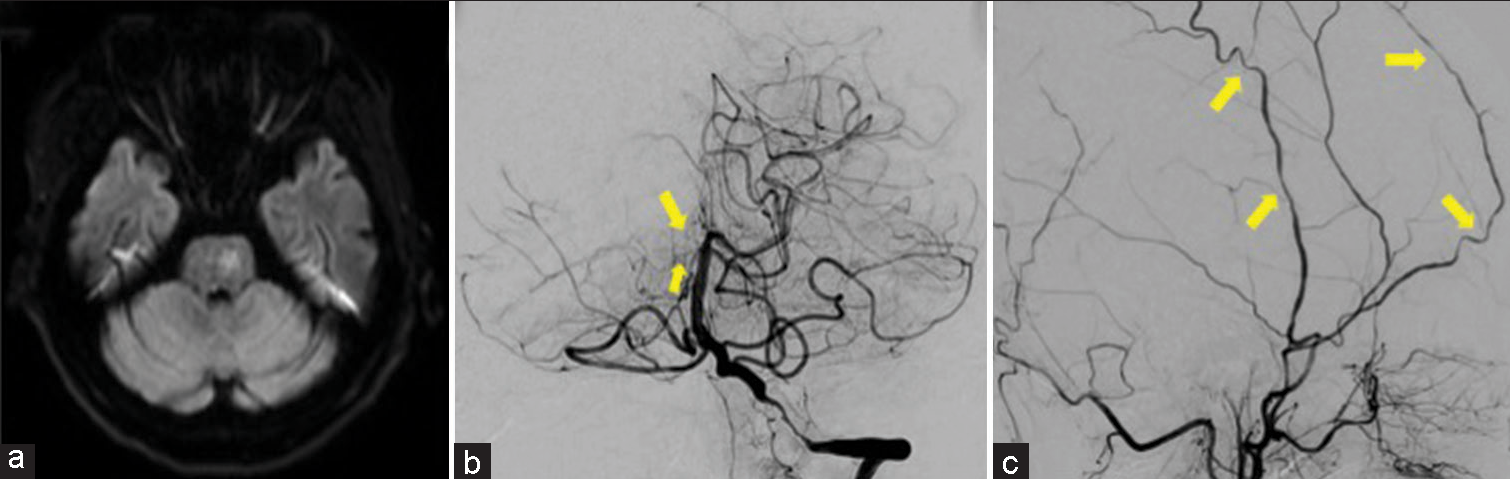
Figure 2:
(a) Diffusion-weighted magnetic resonance imaging revealed a new cerebral infarction in the left pons. (b) In the front view of digital subtraction angiography (DSA), the right posterior cerebral artery and the right superior cerebellar artery showed laminar flow (arrow) due to blood flow through the right posterior communicating artery, and blood flow from the left vertebral artery was predicted to be reduced. (c) The lateral view of DSA revealed a narrowing of the superficial temporal artery at multiple sites (arrow).
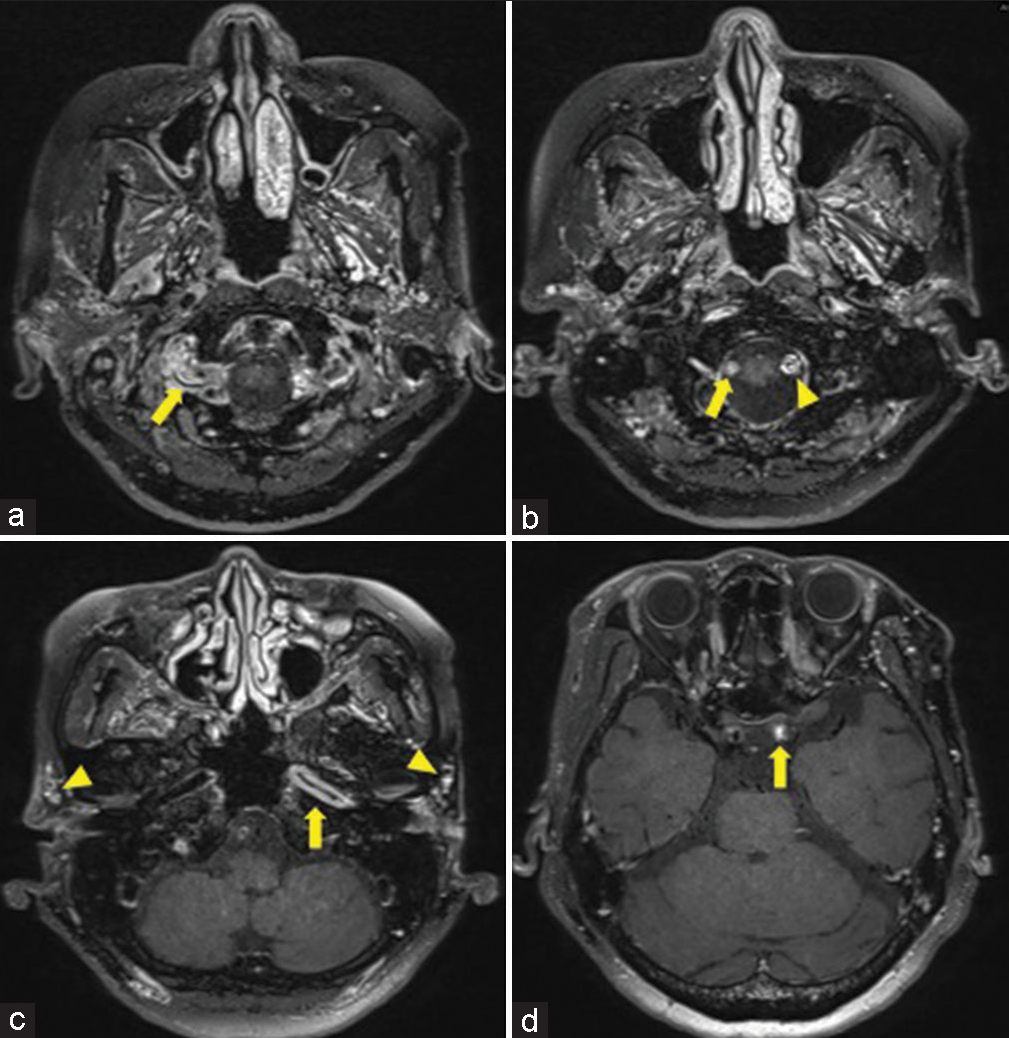
Figure 4:
T1 black-blood post-gadolinium imaging sequence magnetic resonance imaging. (a) Vessel wall enhancement (VWE) of the V4 portion of the right vertebral artery (VA) was observed (arrow). (b) More distally, the right VA was occluded (arrow), and the VWE of the V4 portion of the left VA was observed (triangle). (c) VWE was observed in the petrous portion of the left internal carotid artery (ICA) (arrow), and VWE was also observed in the left and right superficial temporal artery (triangle). (d) Occlusion was observed in the supraclinoid portion of the left ICA (arrow).
Figure 5:
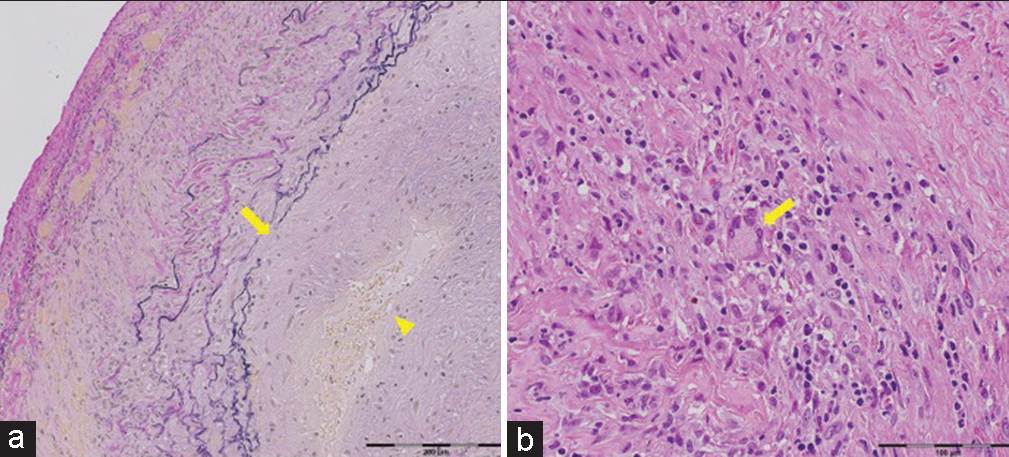
Photographs of pathological sections of the biopsied temporal artery. (a) Elastica van Gieson staining revealed the rupture of the elastic lamina near the intima (arrow) and the narrowing of the vessel lumen due to the marked thickening of the intima (triangle). (b) Hematoxylin and eosin staining revealed multinucleated giant cells in the media (arrow) and infiltration of histiocytes and lymphocytes.
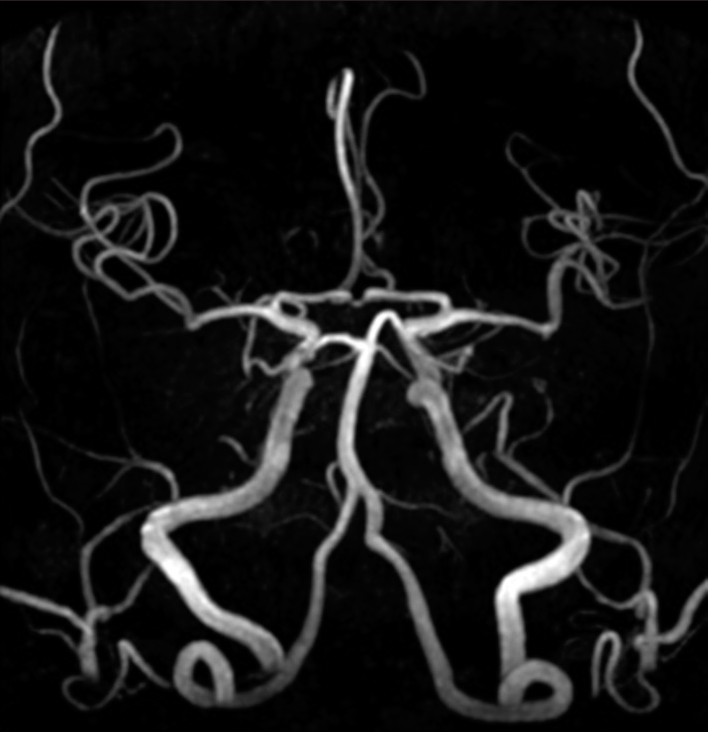
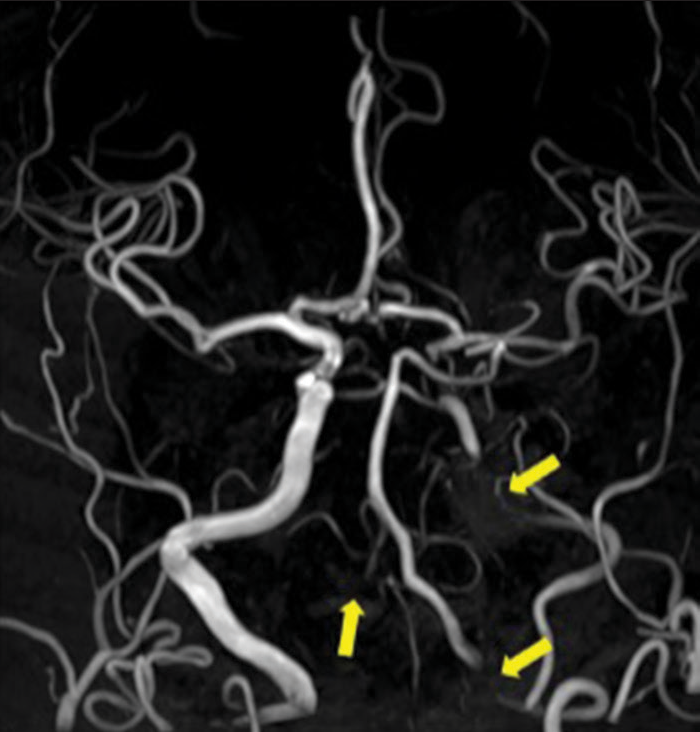
Oral administration of prednisolone 60 mg/day was initiated. Afterward, cerebral infarction did not relapse, the prednisolone dose was gradually reduced, and antiplatelet drugs were changed to aspirin alone. The patient recovered sufficiently to walk independently and was discharged 2 months later. As of 9 months after discharge, the oral prednisolone dose was gradually reduced to 10 mg/day, and MRA showed no aggravated intracranial vascular lesions [
Figure 6:
In the magnetic resonance angiography acquired 9 months after discharge, no progression of intracranial vascular lesions was observed, but the visualization of the left internal carotid artery and the right vertebral artery (VA) did not improve, and the left VA also remained poorly visualized (arrow).
DISCUSSION
Typical pathological findings in GCA are giant cell granulomas formed in the vicinity of destroyed internal elastic lamina and inflammation affecting all the adventitia, media, and intima of arteries.[
It is unknown when the progression of intracranial vascular lesions starts in patients with GCA. According to Alsolaimani et al., the signs of GCA preceded CVA in as many as 64% of cases and occurred concurrently with CVA in 17%, while 19% had no signs of GCA. In addition, there was a median interval of 60 days from the onset of the signs of GCA to the onset of CVA.[
Intracranial vascular lesions found in middle-aged and older patients with cerebral infarction are difficult to determine whether they are arteriosclerotic lesions or vasculitic lesions. In fact, in multiple case reports on intracranial GCA, including the present case, patients were diagnosed with atherothrombotic brain infarction at the onset of cerebral infarction and received standard treatment.[
Unlike extracranial GCA, intracranial GCA has very poor prognoses, even with glucocorticoid or immunosuppressant treatment.[
CONCLUSION
In patients with GCA, the progression of intracranial vascular lesions may begin later than the signs of GCA and continue despite the improvement in the symptoms of GCA. Physicians should be fully aware of the possible delayed formation of intracranial vascular lesions in patients with GCA, and continuous evaluation of intracranial vessels since the onset of GCA may be helpful for early diagnosis at the onset of CVA. In addition, T1 black-blood post-gadolinium imaging sequence MRI may lead to early diagnosis and treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alsolaimani RS, Bhavsar SV, Khalidi NA, Pagnoux C, Mandzia JL, Tay K. Severe intracranial involvement in giant cell arteritis: 5 Cases and literature review. J Rheumatol. 2016. 43: 648-56
2. Calabrese LH, Duna GF, Lie JT. Vasculitis in the central nervous system. Arthritis Rheum. 1997. 40: 1189-201
3. Caselli RJ, Hunder GG, Whisnant JP. Neurologic disease in biopsy-proven giant cell (temporal) arteritis. Neurology. 1988. 38: 352-9
4. Crowson CS, Matteson EL, Myasoedova E, Michet CJ, Ernste FC, Warrington KJ. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 2011. 63: 633-9
5. González-Gay MA, Blanco R, Rodríguez-Valverde V, Martínez-Taboada VM, Delgado-Rodriguez M, Figueroa M. Permanent visual loss and cerebrovascular accidents in giant cell arteritis: Predictors and response to treatment. Arthritis Rheum. 1998. 41: 1497-504
6. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F. 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013. 65: 1-11
7. Larivière D, Sacre K, Klein I, Hyafil F, Choudat L, Chauveheid MP. Extra-and intracranial cerebral vasculitis in giant cell arteritis: An observational study. Medicine (Baltimore). 2014. 93: e265
8. Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008. 58: 26-35
9. Naderi N. Development of intracranial vasculitis in giant cell arteritis during tocilizumab treatment. Clin Exp Rheumatol. 2020. 38: 207-9
10. Parra J, Domingues J, Sargento-Freitas J, Santana I. Extensive intracranial involvement with multiple dissections in a case of giant cell arteritis. BMJ Case Rep. 2014. 2014: bcr2014204130
11. Rodriguez-Régent C, Ben Hassen W, Seners P, Oppenheim C, Régent A. 3D T1-weighted black-blood magnetic resonance imaging for the diagnosis of giant cell arteritis. Clin Exp Rheumatol. 2020. 38: 95-8
12. Salvarani C, Giannini C, Miller DV, Hunder G. Giant cell arteritis: Involvement of intracranial arteries. Arthritis Rheum. 2006. 55: 985-9
13. Sanchez-Alvarez C, Hawkins AS, Koster MJ, Lehman VT, Crowson CS, Warrington KJ. Clinical and radiographic features of giant cell arteritis with intracranial involvement. ACR Open Rheumatol. 2020. 2: 471-7
14. Shigeyasu M, Sasaki N, Nishino S, Sakai N. Giant cell arteritis with simultaneous onset of multiple intracranial vascular occlusions: A case report. Surg Neurol Int. 2022. 13: 21
15. Solans-Laqué R, Bosch-Gil JA, Molina-Catenario CA, Ortega-Aznar A, Alvarez-Sabin J, Vilardell-Tarres M. Stroke and multi-infarct dementia as presenting symptoms of giant cell arteritis: Report of 7 cases and review of the literature. Medicine (Baltimore). 2008. 87: 335-44
16. Wilkinson IM, Russell RW. Arteries of the head and neck in giant cell arteritis. A pathological study to show the pattern of arterial involvement. Arch Neurol. 1972. 27: 378-91
17. Wilkinson IM. The vertebral artery. Extracranial and intracranial structure. Arch Neurol. 1972. 27: 392-6
18. Winkler A, True D. Giant cell arteritis: 2018 review. Mo Med. 2018. 115: 468-70
19. Zarar A, Zafar TT, Khan AA, Suri MF, Qureshi AI. Internal carotid artery stenosis associated with giant cell arteritis: Case report and discussion. J Vasc Interv Neurol. 2014. 7: 24-7