- Department of Neurosurgery, Kobe City Medical Center General Hospital, Kobe, Hyogo, Japan.
- Department of Diagnostic Pathology, Kobe City Medical Center General Hospital, Kobe, Hyogo, Japan.
Correspondence Address:
Masashi Shigeyasu, Departments of Neurosurgery, Kobe City Medical Center General Hospital, Kobe, Hyogo, Japan.
DOI:10.25259/SNI_1001_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masashi Shigeyasu1, Natsuhi Sasaki1, Shogo Nishino2, Nobuyuki Sakai1. Giant cell arteritis with simultaneous onset of multiple intracranial vascular occlusions: A case report. 20-Jan-2022;13:21
How to cite this URL: Masashi Shigeyasu1, Natsuhi Sasaki1, Shogo Nishino2, Nobuyuki Sakai1. Giant cell arteritis with simultaneous onset of multiple intracranial vascular occlusions: A case report. 20-Jan-2022;13:21. Available from: https://surgicalneurologyint.com/surgicalint-articles/11355/
Abstract
Background: Giant cell arteritis (GCA) causes severe stenosis or occlusion of the arteries but rarely affects the intracranial arteries. We report a rare case of GCA along with autopsy results.
Case Description: A 69-year-old man developed gait disturbance due to vertebral artery (VA) occlusion. As is common in atherothrombotic stroke, dual antiplatelet therapy was administered. The patient’s symptoms improved temporarily. However, his symptoms relapsed and his consciousness was acutely disturbed. Digital subtraction angiography revealed an appearance of stenosis of the internal carotid artery (ICA) C2 portion on the right side and decreased retrograde basilar artery (BA) blood flow through the right posterior communicating artery. Balloon angioplasty was performed, and BA blood flow increased. GCA was suspected, and a definitive diagnosis was made based on temporal artery biopsy findings. Steroid therapy was initiated but failed to control disease progression, and the patient died. The autopsy findings revealed GCA in the bilateral ICAs and VAs, and no signs of GCA were found in other intracranial arteries, despite occlusion on magnetic resonance angiography.
Conclusion: GCA of the intracranial blood vessels is rare and might be more likely to occur in the ICAs and VAs than in other intracranial blood vessels. GCA of the intracranial blood vessels has a poor prognosis, and as such, if rapid changes are observed in the ICAs or VAs, GCA should be considered a part of the differential diagnosis and immediate treatment should be administered.
Keywords: Angioplasty, Autopsy, Giant cell arteritis, Multiple intracranial vascular occlusions
INTRODUCTION
Giant cell arteritis (GCA) is a chronic granulomatous vasculitis of the large- and medium-sized arteries, and predominantly affects the extracranial arteries.[
CASE DESCRIPTION
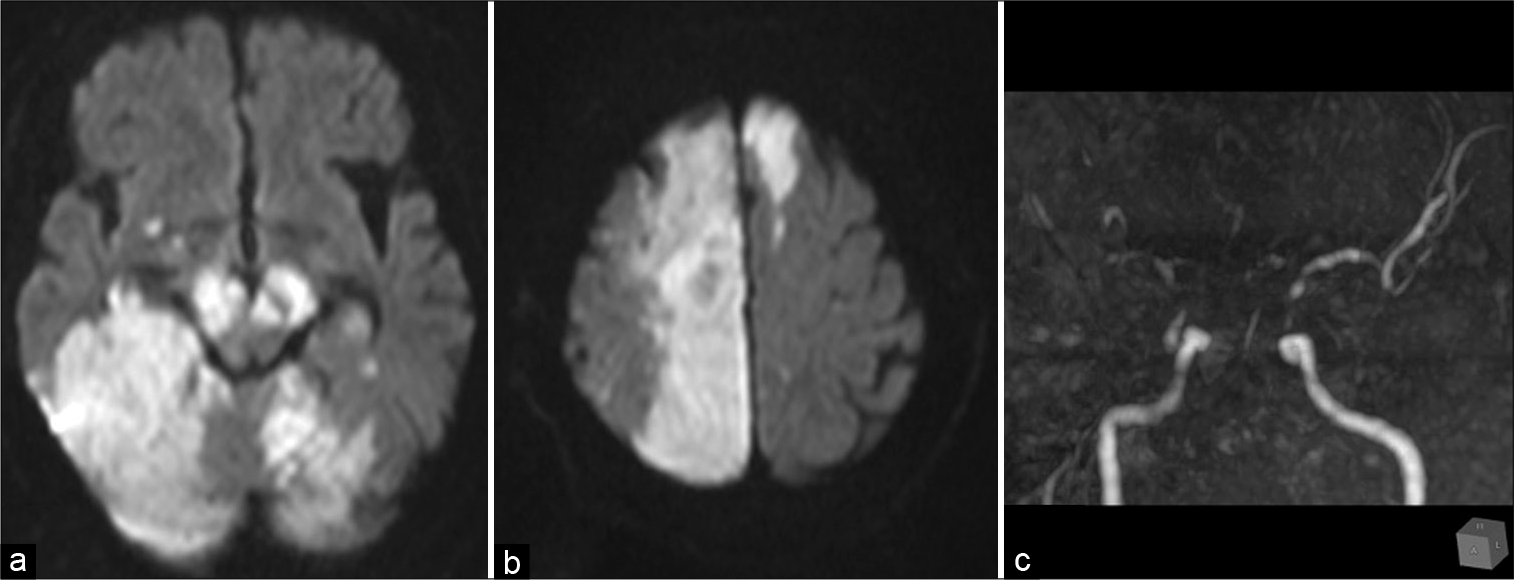
A 69-year-old man with a 1-month history of intermittent headaches and dizziness was transferred to our hospital due to a sudden onset of gait disturbance. The patient had untreated mild hypertension. Physical examination showed ataxia, but no other neurological deficits were noted. He also had a sore throat and his body temperature was 37.5°C. Magnetic resonance imaging (MRI) showed acute ischemic infarctions on both sides of the cerebellum [
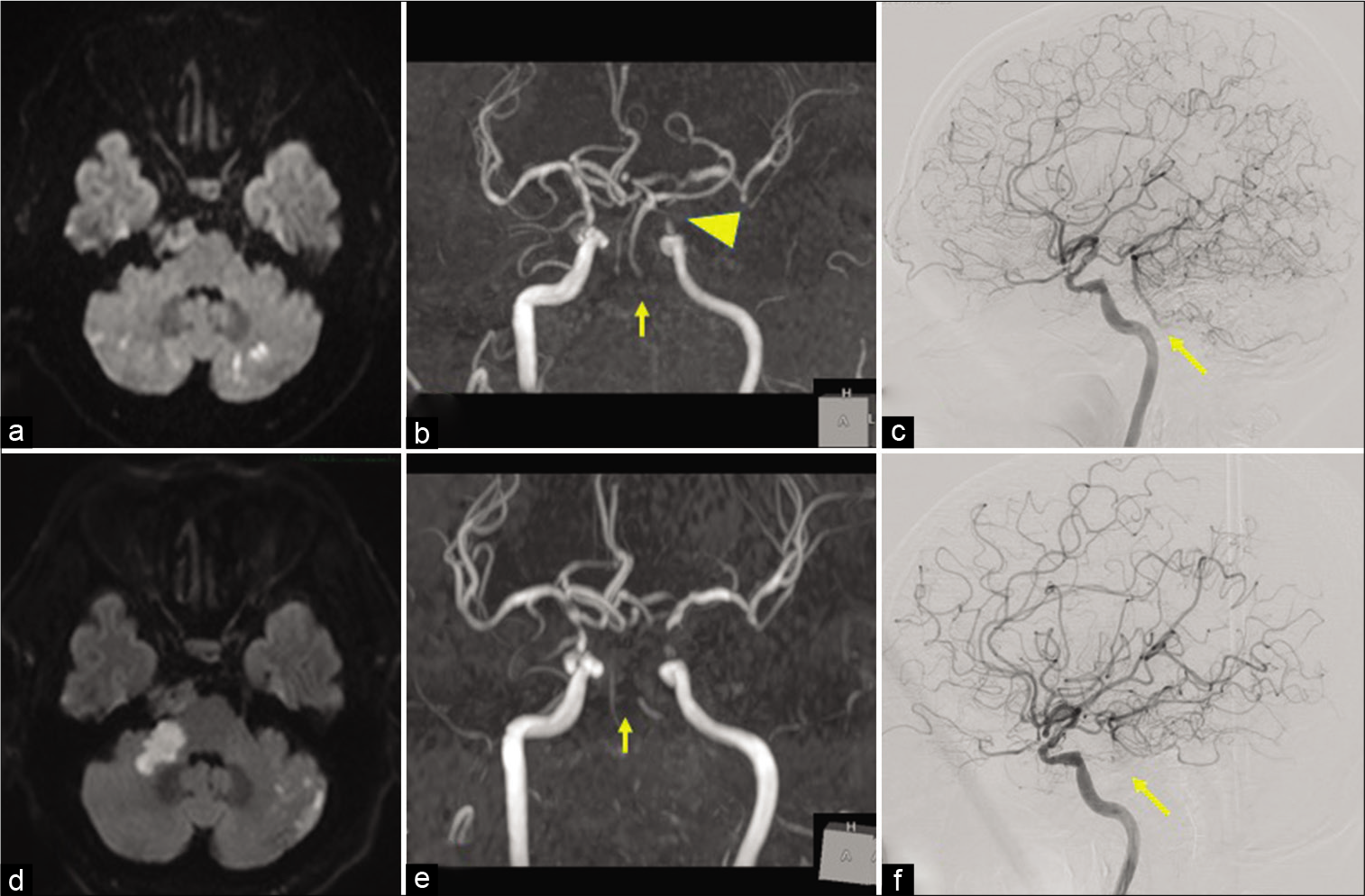
Figure 1:
(a) Diffusion-weighted MRI showed bilateral cerebellar infarctions, and (b) MRA showed the left ICA stenosis (arrowhead) and the complete occlusions of the bilateral vertebral arteries (arrow) on admission. (c) Lateral view of the angiography showed retrograde filling of the BA and AICA from the right PCoA (arrow) on admission. (d) Diffusion-weighted MRI showed infarctions in the right cerebellar peduncle and the left cerebellar hemisphere, and (e) MRA showed poor visualization of the BA. (f) Lateral view of the angiography showed a decline in retrograde BA blood flow (arrow) compared to the image at admission. AICA: Anterior inferior cerebellar artery, BA: Basilar artery, ICA: Internal carotid artery, MRI: Magnetic resonance imaging, MRA: Magnetic resonance angiography, PCoA: Posterior communicating artery.
After rehabilitation, the symptoms of cerebellar ataxia improved and he was discharged on day 21. At discharge, clopidogrel was changed to cilostazol (200 mg/day) as the latter has a lower risk of bleeding complications. However, dysarthria and gait disorders relapsed on day 32, and the patient was readmitted. The MRI showed diffuse cerebral infarctions in the right cerebellar peduncle and the left cerebellar hemisphere. DSA detected a decline in retrograde BA blood flow, which resulted in obstruction in the right anterior inferior cerebellar artery [
On day 36, his consciousness was acutely disturbed, and MRI and DSA were urgently performed. DSA revealed an appearance of stenosis of the ICA C2 portion on the right side and decreased retrograde BA blood flow through the right PCoA, which was not observed at his first admission [
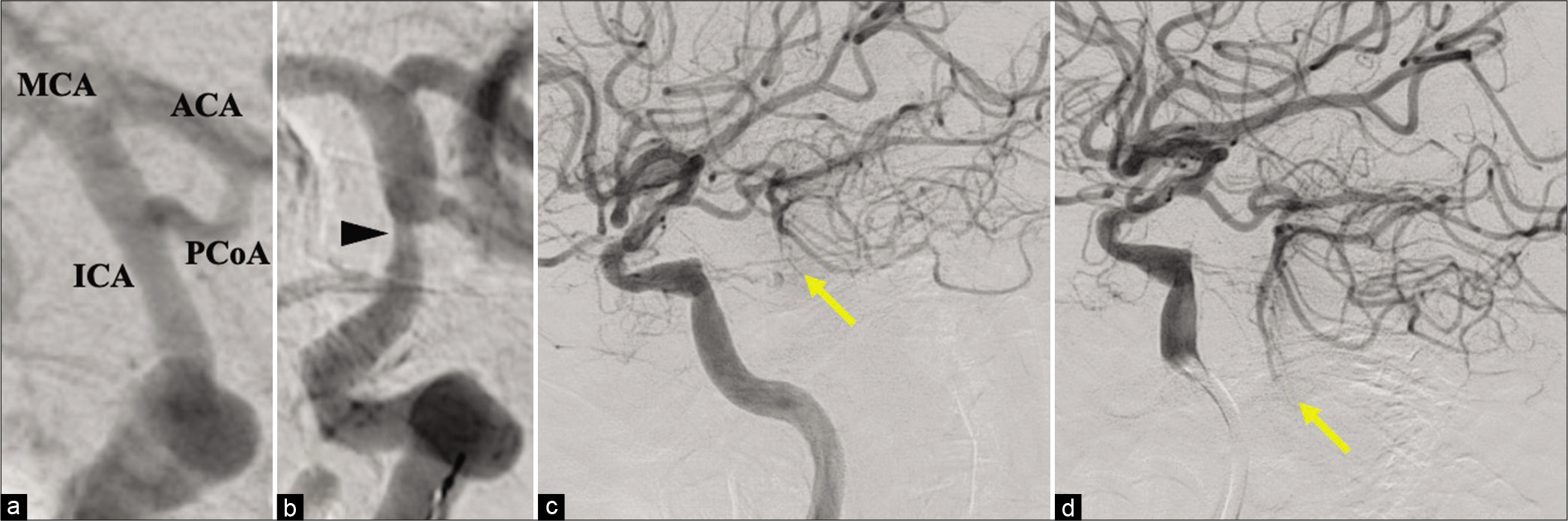
Figure 2:
(a and b) Anteroposterior views of the cerebral angiography. (a) The right ICA at the C2 portion on admission. There was no sign of stenosis, (b) but severe stenosis appeared after his level of consciousness was decreased. (c and d) Lateral projections of the cerebral angiography of the right ICA. (c) Before angioplasty, (d) after angioplasty. Note that retrograde flow in the BA through the PCoA due to the bilateral VA occlusion was improved (arrow) because of the vasodilation of the right ICA. ACA: Anterior cerebral artery, BA: Basilar artery, ICA: Internal carotid artery, MCA: Middle cerebral artery, PCoA: Posterior communicating artery, VA: Vertebral artery.
Autopsy result
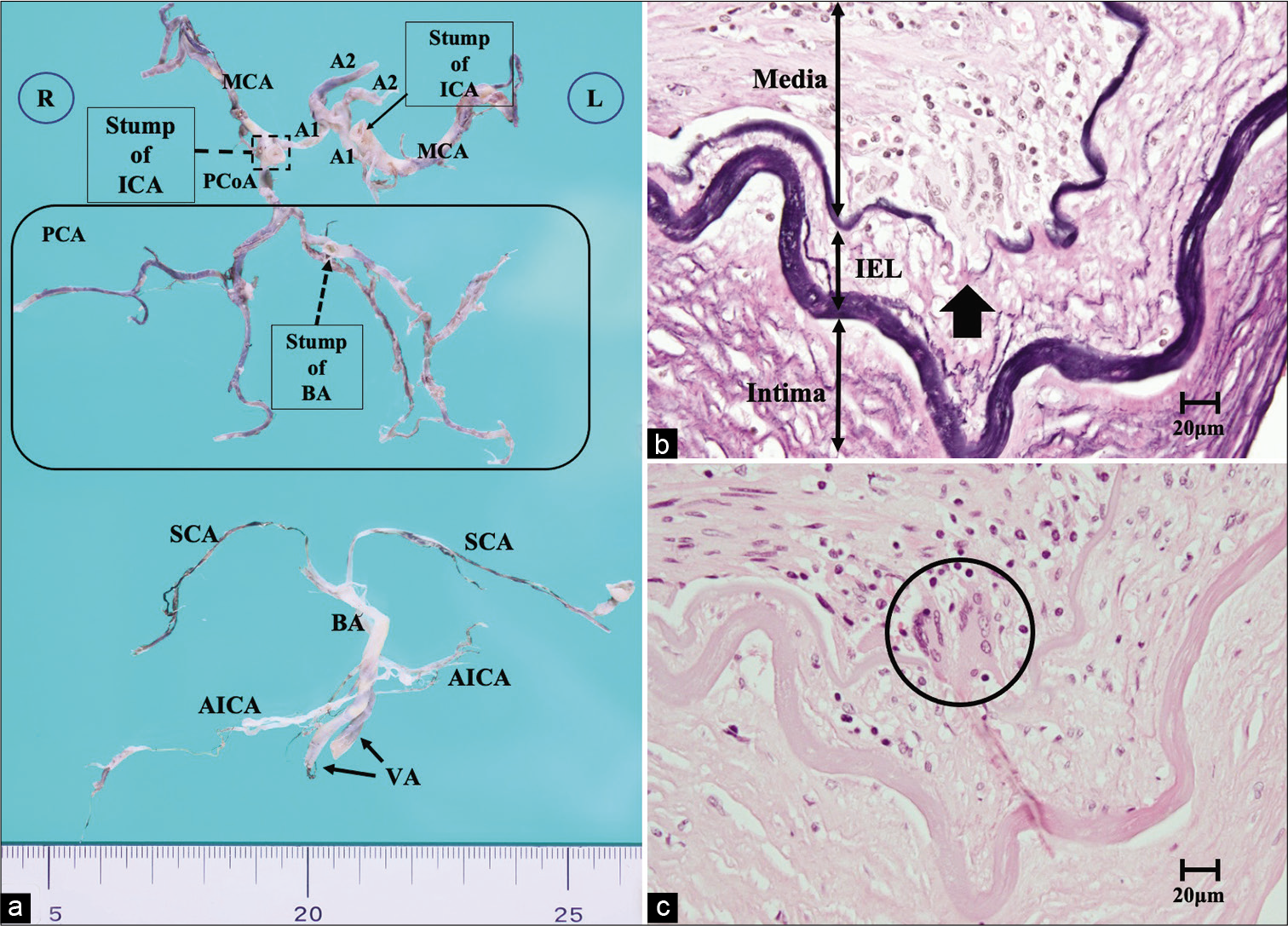
The autopsy revealed cerebral infarctions in the lower right medulla and reticulum, which were thought to be the cause of respiratory depression. Microscopically, internal elastic lamina (IEL) disruptions with giant cells, granuloma, as well as transmural inflammatory infiltrates were observed in the bilateral ICAs and VAs [
Figure 4:
(a) Macroscopic photograph of the intracranial arteries. The left PCoA was hypoplasia, and the bilateral ICAs were cut at the C2 portion, and the BA was disconnected at the top. (b and c) Microscopic sections of the right ICA at the C2 portion. (b) Elastica van Gieson staining ×400, (c) hematoxylin and eosin staining ×400. All layers of the arterial wall are severely affected by the arteritis, and multinucleated giant cells invade the junction of the media and intima (circle). The IEL was also destroyed (arrow). A1: Anterior cerebral artery A1 segment, A2: Anterior cerebral artery A2 segment, AICA: Anterior inferior cerebellar artery, BA: Basilar artery, IEL: Internal elastic lamina, ICA: Internal carotid artery, MCA: Middle cerebral artery, PCoA: Posterior communicating artery, PCoA: Posterior communicating artery, SCA: Superior cerebellar artery, VA: Vertebral artery.
DISCUSSION
GCA predominantly affects the branches of the aorta, especially the branches of the ophthalmic and external carotid arteries, and rarely involves cerebral infarction.[
According to the past reports involving inflammation of intracranial blood vessels, vasculitis progresses despite prednisolone administration, and the prognosis is often poor.[
CONCLUSION
We describe a case of GCA with extensive cerebral and brainstem infarction due to progressive stenoses of multiple intracranial arteries. GCA of the intracranial blood vessels is rare and might be more likely to occur in the ICAs and VAs than in other intracranial blood vessels. If multiple rapid changes in the ICA and VA are observed, GCA needs to be differentiated and immediate treatment is required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alsolaimani RS, Bhavsar SV, Khalidi NA, Pagnoux C, Mandzia JL, Tay KY. Severe intracranial involvement in giant cell arteritis: 5 Cases and literature review. J Rheumatol. 2016. 43: 648-56
2. Bogousslavsky J, Deruaz JP, Regli F. Bilateral obstruction of internal carotid artery from giant-cell arteritis and massive infarction limited to the vertebrobasilar area. Eur Neurol. 1985. 24: 57-61
3. Calabrese LH, Duna GF, Lie JT. Vasculitis in the central nervous system. Arthritis Rheum. 1997. 40: 1189-201
4. Chen ZW, Symons SP, Young B, Bilbao JM. A 24-year-old male with headaches. Brain Pathol. 2010. 20: 863-6
5. Cull RE. Internal carotid artery occlusion caused by giant cell arteritis. J Neurol Neurosurg Psychiatry. 1979. 42: 1066-7
6. Dementovych N, Mishra R, Shah QA. Angioplasty and stent placement for complete occlusion of the vertebral artery secondary to giant cell arteritis. J Neurointerv Surg. 2012. 4: 110-3
7. Gailloud P, Clatterbuck RE, Fasel JH, Tamargo RJ, Murphy KJ. Segmental agenesis of the internal carotid artery distal to the posterior communicating artery leading to the definition of a new embryologic segment. AJNR Am J Neuroradiol. 2006. 27: 246-7
8. García-garcía J, Ayo-Martín Ó, Argandoña-Palacios L, Segura T. Vertebral artery halo sign in patients with stroke: A key clue for the prompt diagnosis of giant cell arteritis. Stroke. 2011. 42: 3287-90
9. Guerrero AM, Sierra-Hidalgo F, Calleja P, Navia P, Campollo J, Díaz-Guzmán J. Intracranial internal carotid artery angioplasthy and stenting in giant cell arteritis. J Neuroimaging. 2015. 25: 307-9
10. Haughton VM, Newton TM, Potts DG.editors. The normal and anomalous aortic arch and brachiocephalic arteries. Radiol Skull Brain. Missouri, United States: Mosby; 1974. 2: 1145-63
11. Larivière D, Sacre K, Klein I, Hyafil F, Choudat L, Chauveheid MP. Extra-and intracranial cerebral vasculitis in giant cell arteritis. Medicine (Baltimore). 2014. 93: e265
12. McLean CA, Gonzales MF, Dowling JP. Systemic giant cell arteritis and cerebellar infarction. Stroke. 1993. 24: 899-902
13. Salvarani C, Cantini F, Boiardi L, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med. 2002. 347: 261-71
14. Salvarani C, Giannini C, Miller DV, Hunder G. Giant cell arteritis: Involvement of intracranial arteries. Arthritis Rheum. 2006. 55: 985-9
15. Sanchez-Alvarez C, Hawkins AS, Koster MJ, Lehman VT, Crowson CS, Warrington KJ. Clinical and radiographic features of giant cell arteritis with intracranial involvement. ACR Open Rheumatol. 2020. 2: 471-7
16. Sheehan MM, Keohane C, Twomey C. Fatal vertebral giant cell arteritis. J Clin Pathol. 1993. 46: 1129-31
17. Solans-Laqué R, Bosch-Gil JA, Molina-Catenario CA, Ortega-Aznar A, Alvarez-Sabin J, Vilardell-Tarres M. Stroke and multi-infarct dementia as presenting symptoms of giant cell arteritis: Report of 7 cases and review of the literature. Medicine (Baltimore). 2008. 87: 335-44
18. Wilkinson IM, Russell RW. Arteries of the head and neck in giant cell arteritis: A pathological study to show the pattern of arterial involvement. Arch Neurol. 1972. 27: 378-91
19. Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL. The American college of rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum. 1990. 33: 160-72