- Department of Neurosurgery, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India
- Department of Pathology and Laboratory Medicine, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India
Correspondence Address:
Kavindra Singh, Department of Neurosurgery, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India.
DOI:10.25259/SNI_713_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kavindra Singh1, Rahul Singh1, Rakesh K. Sihag2, Arvind Kumar2. Giant cerebellar neurocysticercosis masquerading a primary central nervous system neoplasm – A case report with review of literature. 15-Nov-2024;15:416
How to cite this URL: Kavindra Singh1, Rahul Singh1, Rakesh K. Sihag2, Arvind Kumar2. Giant cerebellar neurocysticercosis masquerading a primary central nervous system neoplasm – A case report with review of literature. 15-Nov-2024;15:416. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13229
Abstract
Background: Neurocysticercosis (NCC) is one of the leading parasitic infections of the brain. Giant NCC is rare, with only two cases of cerebellar involvement reported till now. In the presence of a host immune response, these giant NCCs can mimic primary central nervous system neoplasms. The objective of this article is to report a rare case of giant cerebellar NCC and its management strategy with a literature review.
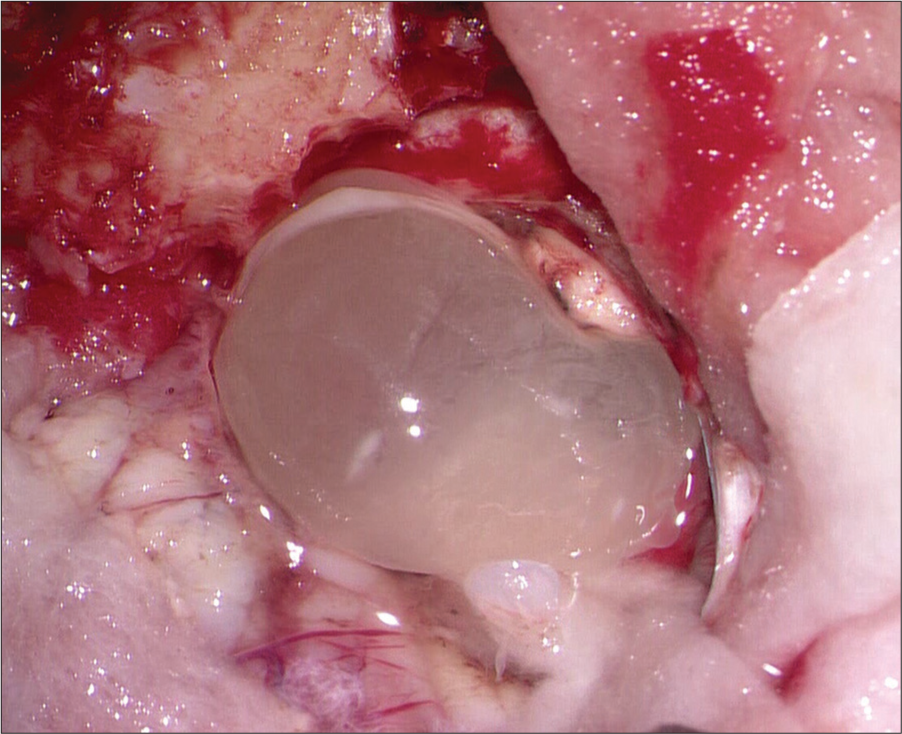
Case Description: A young male presented with a giant cerebellar ring-enhancing mass with features of raised intracranial pressure, and surgical excision was done. The patient made an uneventful recovery.
Conclusion: Surgical excision is safe for NCC, especially in the presence of a diagnostic dilemma.
Keywords: Central nervous system neoplasms, Colloidal vesicular, Neurocysticercosis, Ring-enhancing lesion
INTRODUCTION
Neurocysticercosis (NCC) is a parasitic infection of the brain caused by the larval forms of the cestode Taenia solium. It is a common cause of new-onset seizures in the adult population.[
CASE REPORT
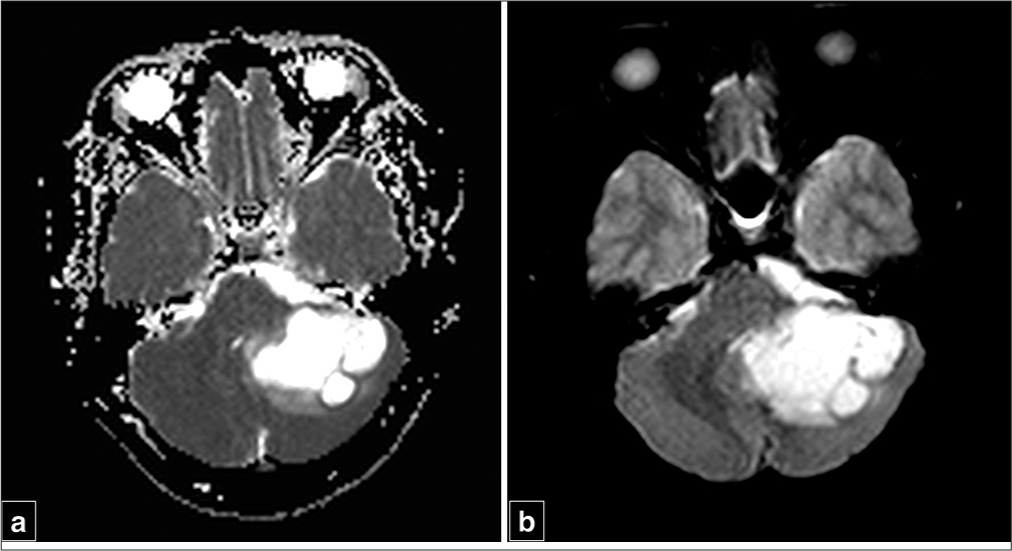
A 32-year-old gentleman came to our outpatient clinic with complaints of holocranial headache of increasing severity for the past 2 months, along with swaying while walking. Examination revealed positive cerebellar signs with no evidence of papilledema. On CEMRI brain, there was a 4.8 × 3.8 × 2.8 cm involving the left cerebellum, and it was hypointense on T1, hyperintense on T2 along with ring-like contrast enhancement with the presence of perilesional edema in the form of fluid-attenuated inversion recovery (FLAIR) hyperintensities adjacent to the lesion [
Figure 1:
(a) Noncontrast computed tomography head showing a hypodense lesion in left cerebellum, (b-d) Axial magnetic resonance imaging (MRI) T1-weighted sequence showing a hypointense lesion involving left cerebellar hemisphere with hyperintense signal on T2-weighted sequences matching that of cerebrospinal fluid along with a fluid-attenuated inversion recovery hypointensity with surrounding hyperintense signal suggestive of perilesional edema, (e and f) Axial T1-weighted image with contrast sequences, (g) Sagittal and (h) coronal T1-weighted sequences with contrast depicting a ring enhancing lesion in the left cerebellum.
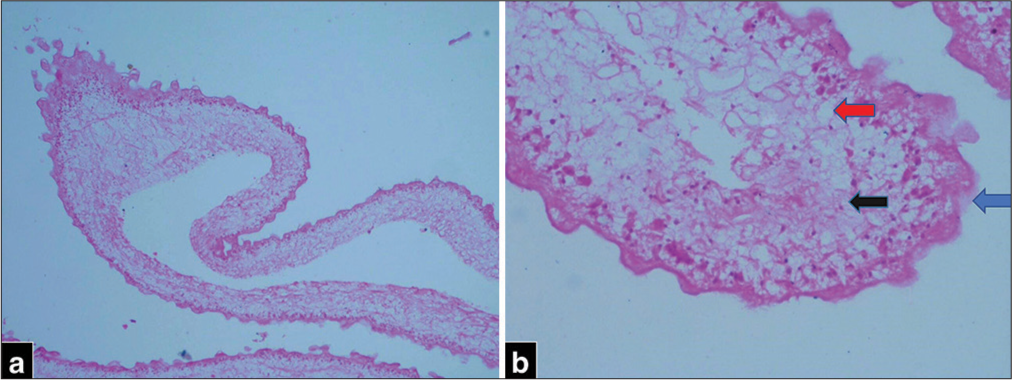
Pathological examination revealed a cyst wall with 3 layers [
DISCUSSION
The first reported case of NCC dates back to 1558 when Rumler noticed fluid-filled vesicles adherent to the meninges while performing an autopsy of a patient with epilepsy.[
NCC includes a spectrum of diseases that differ in pathogenesis and clinical features.[
Parenchymal NCC
Nonviable calcified Single small enhancing lesion Viable parenchymal
Extraparenchymal NCC
Intraventricular Subarachnoid Spinal
Imaging plays an important role in the diagnosis of NCC. The characteristic imaging findings of NCC in various stages have been described in great detail.[
The diagnosis of NCC is mostly based on imaging alone and serologic tests such as enzyme-linked immunotransfer blot or enzyme-linked immunosorbent assay are done to confirm the diagnosis once suspected on imaging.[
Sabel et al., in 2001, first reported a case of a 47-year-old male with a short history of speech difficulties.[
A common link in all the reported and present cases is the presence of a large parenchymal NCC (>20 mm) with peripheral ring contrast enhancement and perilesional edema and mass effect. Most of the reported cases, along with the current case, belong to the colloidal vesicular stage of pathological staging, where a host reaction will lead to the formation of a pseudocapsule, which can take contrast enhancement in the form of a ring, hence mimicking intrinsic CNS neoplasms such as cystic glioma.
To our knowledge, a large NCC (>2 cm) presenting in the infratentorial compartment has never been reported in the literature. This is the first report of a giant cerebellar NCC presenting with mass effect. Advanced imaging modalities such as spectroscopy can be of some help, but in cases of raised ICP features along with a diagnostic dilemma, surgery can be done with favorable outcomes, as is evident from the above table. Medical management, including antihelminthic drugs along with a tapering dose of steroids, remains the mainstay of treatment in the majority of the cases.
CONCLUSION
NCC, a common parasitic infestation of CNS, can sometimes mimic CNS neoplasms, especially when large. Surgical excision in patients with raised ICP and having diagnostic dilemmas has shown favorable outcomes.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bouillot S, Monteil P, Dautheribes M, Rougier A, Guerin J, Vital A. Deux cas de neurocysticercose intracérébrale à révélation pseudo-tumorale [Two cases of neurocysticercosis mimicking brain tumor]. Ann Pathol. 2003. 23: 355-7
2. Brutto OH, García HH. Taenia solium cysticercosis--The lessons of history. J Neurol Sci. 2015. 359: 392-5
3. Chawla S, Asadollahi S, Gupta PK, Nath K, Brem S, Mohan S. Advanced magnetic resonance imaging and spectroscopy in a case of neurocysticercosis from North America. Neuroradiol J. 2022. 35: 119-25
4. Kim JH, Suh SI, Kim JH, Kwon TH, Chung HS. Giant neurocysticercosis cyst in the cerebellar hemisphere. Neurol Med Chir (Tokyo). 2006. 46: 412-4
5. Kim SW, Kim MK, Oh SM, Park SH. Racemose cysticercosis in the cerebellar hemisphere. J Korean Neurosurg Soc. 2010. 48: 59-61
6. Rajshekhar V. Neurocysticercosis: Diagnostic problems & current therapeutic strategies. Indian J Med Res. 2016. 144: 319-26
7. Sabel M, Neuen-Jacob E, Vogt C, Weber F. Intracerebral neurocysticercosis mimicking glioblastoma multiforme: A rare differential diagnosis in Central Europe. Neuroradiology. 2001. 43: 227-30
8. Sevin IE, Kızmazoğlu C, Güvenç G, Ermete M, Yüceer N. Neurocysticercosis as a single lesion mimicking glial tumor. J Neurol Sci Turk. 2017. 34: 189-93
9. Soejitno A, Niryana IW, Sriwidyani NP, Susilawathi NM, Witari NP, Sudewi AA. Neurocysticercosis presented as a solitary cystic parenchymal lesion mimicking primary brain tumor: A case report. IDCases. 2020. 22: e01004
10. Umredkar A, Singla N, Mohindra S, Bal A, Gupta SK. Giant intraparenchymal neurocysticercosis: Report of surgical aspects two cases. Neurol India. 2009. 57: 800-2
11. Vasiljević-VučKović V, Medenica SM, Grujičić D. Neurocysticercosis mimicking brain tumor. Neuroradiol J. 2011. 24: 419-23
12. White AC, Coyle CM, Rajshekhar V, Singh G, Hauser WA, Mohanty A. Diagnosis and treatment of neurocysticercosis: 2017 Clinical practice guidelines by the infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2018. 66: e49-75