- Department of Neurosurgery, Loma Linda University Medical School, Loma Linda, California, United States
- Department of Neurosurgery, Loma Linda University Medical Center, Loma Linda, California, United States.
Correspondence Address:
Miguel Angel Lopez-Gonzalez, Department of Neurosurgery, Loma Linda University Medical Center, Loma Linda, California, United States.
DOI:10.25259/SNI_647_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hannah Choi1, Jorrdan N. R. Bissell1, Brandon Michael Edelbach1, Joel Paea1, Emmanuel Omosor1, Ravi Raghavan1, Vadim Gospodarev2, Miguel Angel Lopez-Gonzalez2. Giant primary intracranial multi-fossa leiomyosarcoma involving the frontal sinus, ethmoid air cells, anterior fossa, middle fossa, and intraventricular space: A case report and literature review. 27-Oct-2023;14:384
How to cite this URL: Hannah Choi1, Jorrdan N. R. Bissell1, Brandon Michael Edelbach1, Joel Paea1, Emmanuel Omosor1, Ravi Raghavan1, Vadim Gospodarev2, Miguel Angel Lopez-Gonzalez2. Giant primary intracranial multi-fossa leiomyosarcoma involving the frontal sinus, ethmoid air cells, anterior fossa, middle fossa, and intraventricular space: A case report and literature review. 27-Oct-2023;14:384. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12610
Abstract
Background: Leiomyosarcomas (LMSs) is a type of sarcoma that arises from smooth muscle and generally presents in the abdomen. Although intracranial LMS has been identified before, most reported presentations have been in immunocompromised patients. Here, we present an intracranial LMS in an immunocompetent patient.
Case Description: A 22-year-old male with a history of an atypical pineal parenchymal tumor of intermediate differentiation resected by suboccipital craniotomy at the age of 12 followed by adjuvant radiation therapy, presented with 3 weeks of decreased appetite, weight loss, and lethargy. He subsequently underwent transbasal approach skull base tumor resection. Histologic examination of the mass along with the patient’s history of radiation was supportive of a low-grade, radiation-induced LMS arising from the anterior fossa of the skull or meninges and extends to the frontal sinus and ethmoid air cells.
Conclusion: Primary intracranial LMS is an extremely rare diagnosis and presenting symptoms vary with the location and size of the tumor. Due to the poor specificity of clinical symptoms, diagnosis is often based on histology. The most common treatment is surgical resection. Adjuvant chemotherapy with various agents has been found to be somewhat effective outside the central nervous system. When LMS does occur, a history of immunocompromised state or previous radiation exposure is often present. Pathological confirmation is required for an appropriate diagnosis.
Keywords: Anterior fossa, Ethmoid air cells, Frontal sinus, Leiomyosarcoma, Middle fossa, Transbasal approach
INTRODUCTION
Leiomyosarcoma (LMS) is a rare subtype of soft-tissue sarcoma that originates from smooth muscle cells.[
The incidence of LMS increases with age, peaking in the seventh decade of life. There are no clear predisposing factors for the development of LMS, although patients with hereditary retinoblastoma or TP53 mutations are at risk. Histologically, LMS is characterized by intersecting, sharply marginated fascicles of spindle cells with abundant eosinophilic cytoplasm and elongated, hyperchromatic nuclei. These features are indicative of the smooth muscle lineage from which these tumors arise. Treatment options for LMS include surgery, radiation therapy, and chemotherapy.[
The incidence of LMS in the central nervous system (CNS), specifically intracranial LMS, is extremely rare. Most cases of intracranial LMS are secondary, meaning they have metastasized or spread from tumors located elsewhere in the body.[
Intracranial LMS has been reported in the literature, but most cases have been identified in immunocompromised patients, particularly those with human immunodeficiency virus (HIV).[
In this study, we present a case of low-grade, radiation-induced LMS arising from the anterior skull base in a patient with a history of an atypical pineal parenchymal tumor of intermediate differentiation. This case adds to the limited literature on primary intracranial LMS and may serve as a potential reference for clinicians and clinical studies.
CASE DESCRIPTION
A 22-year-old male presented with a history of atypical pineal parenchymal tumor of intermediate differentiation resected by suboccipital craniotomy at the age of 12 with adjuvant radiation and chemotherapy, followed by a large, atypical meningioma resected through the left parietal craniotomy at the age of 14. The patient reported 3 weeks of altered mental status, visual deficits, fatigue, decreased appetite, weight loss, and lethargy.
On physical examination, he had dysarthria with the paucity of speech without receptive or expressive aphasia – there was some difficulty with repeating complex sentences. There were no focal neurological deficits, the sensation was intact, and there was full strength and spontaneous movement of all extremities. The patient was afebrile and preliminary laboratory results were unremarkable.
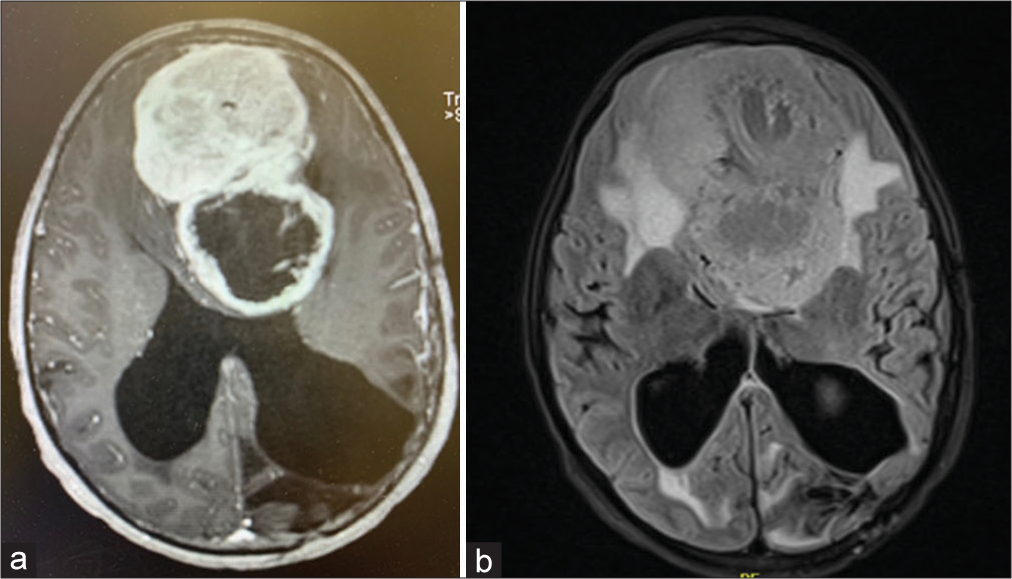
Imaging showed a giant – 9.7 cm anteroposterior × 7.5 cm transverse × 6.6 cm craniocaudal – bilobed, heterogeneous, partially cystic mass centered in the anterior cranial fossa, extending to the middle fossa, and intraventricularly with marked mass effect including regional erosion and involvement of the ethmoid air cells and frontal sinus [
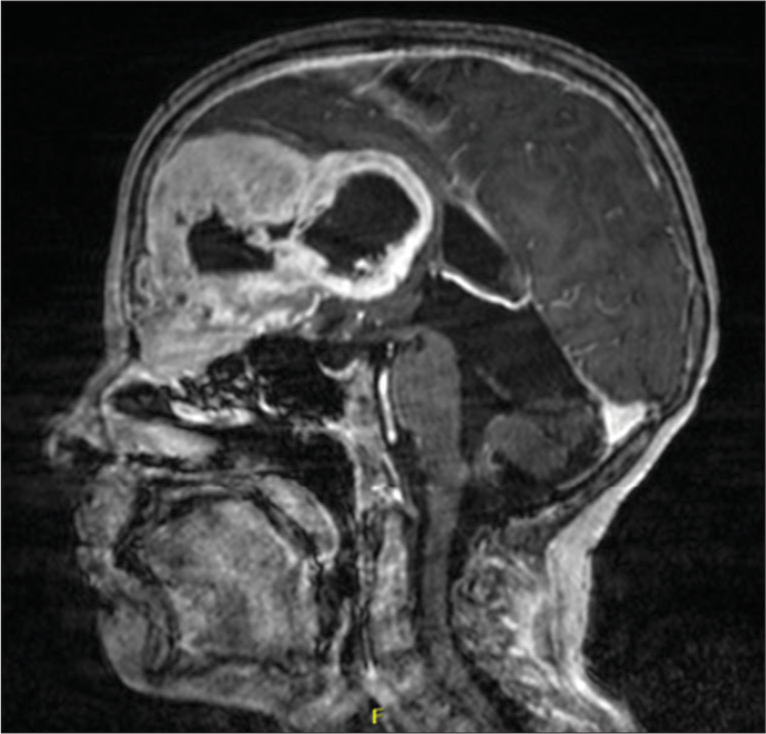
Figure 2:
Sagittal magnetic resonance imaging with contrast showing a large mass centered on the left side of anterior cranial fossa/anterior falx with invasion of the skull base, medial left orbit, and left frontal sinus. There was notable erosion of the lamina papyracea on the left with extensive regional mass effect and effacement of the bilateral frontal horns and possible entrapment/developing hydrocephalus of the right occipital horn.
Given the patient’s history, differential diagnostic considerations included atypical/malignant radiation-induced meningioma or metastatic pineal parenchymal tumor. He subsequently underwent extensive tumor resection through bifrontal craniotomy with a skull base approach to the left orbit and ethmoid sinus for microsurgical resection.
Surgical description
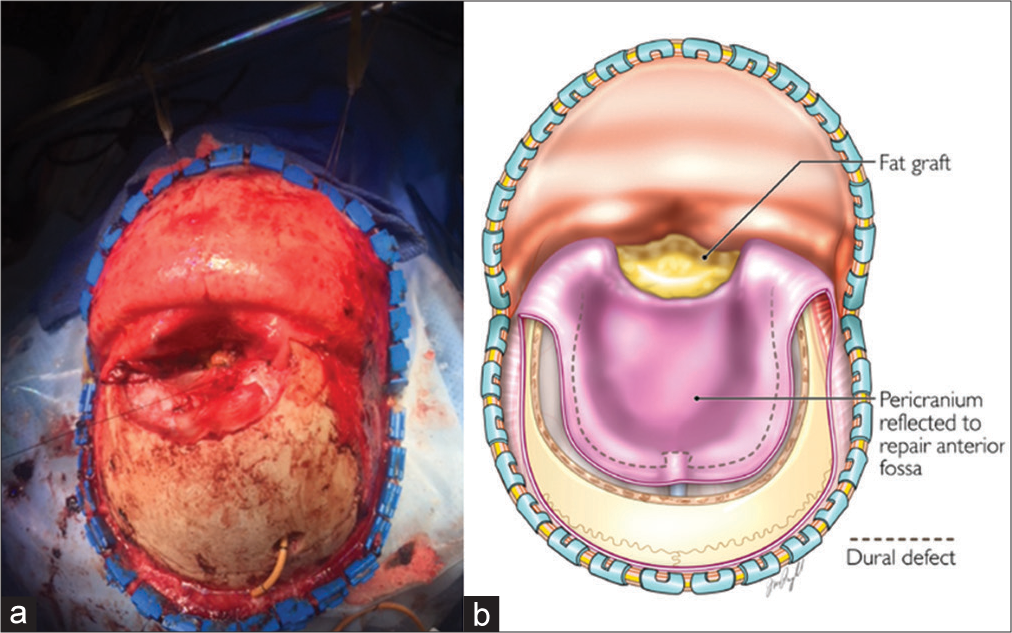
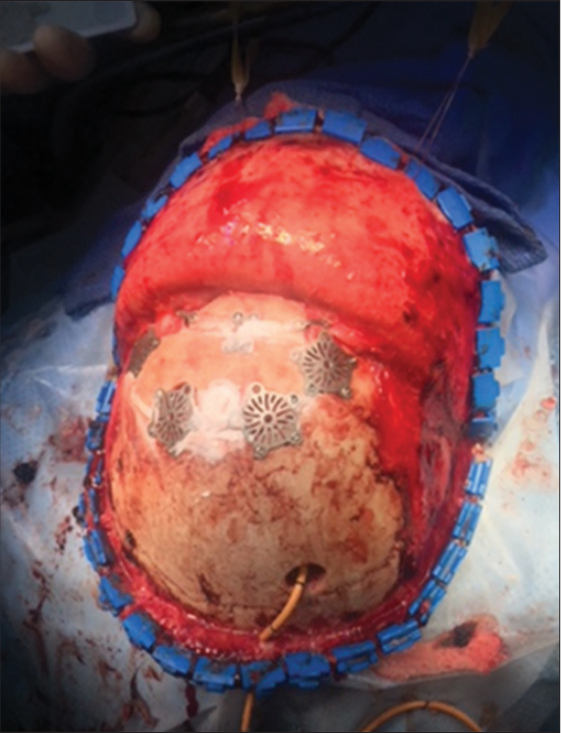
A right parietal ventriculostomy was placed and a bicoronal incision was done for a bifrontal stereotactic craniotomy with pericranium preservation. The bifrontal craniotomy was done with multiple bur holes, one above the glabella, two frontal bilateral, and then intermediate around the superior sagittal sinus. Afterward, the epidural dissection was performed, and the bone flap was removed. An extended transbasal approach was, then, added. The tumor was extending toward the ethmoid sinus and into the left orbit. Using bipolar electrocoagulation, the tumor was devascularized from the anterior and posterior ethmoid arteries bilaterally. We proceeded to debulk the orbital segment of the tumor, followed by the ethmoidal segment, and then finally the dura and anterior fossa. The anterior aspect of the superior sagittal sinus was ligated just behind the tumor using silk sutures as well as hemo-clips. Debulking was done with the ultrasonic aspirator. After adequate central debulking, we were able to access the posterior lobule of the tumor and collapsed the capsule, dissecting it away from the third ventricle and lateral ventricle. When approaching the segment of the tumor that was attached to the pericallosal artery on the right side, a small opening of the pericallosal artery was encountered. A temporary clip was used proximally, and (9 0) sutures were placed to close the area of hemorrhage adequately. The temporary clip was removed, and adequate flow was restored after this. Hemostasis was carefully achieved. An absorbable hemostatic sponge and surgical glue were used to isolate the ventricle from the surgical cavity in the anterior fossa. An additional ventriculostomy was placed with direct visualization. We used a fat graft that was harvested from the abdomen to seal the anterior fossa around the ethmoid sinus and the orbit. We placed fibrin glue and then a titanium plate was used to reconstruct the anterior aspect of the floor of the anterior fossa. We continued to perform the suturing of the pericranium down to the dura at the level of the titanium mesh. At this point, we placed a nonsuturable dura substitute and fibrin glue followed by a suturable dura substitute in the convexity [
Pathology
Histologic examination of the mass revealed a spindle-cell neoplasm with fasciculate architecture and strong diffuse reactivity to caldesmon, supporting smooth vessel origins. The tumor specimen was negative for STAT-6 and e-cadherin staining. These findings, along with the patient’s history of radiation, were supportive of an LMS arising from the base of the skull or meninges.
Outcome
The patient required a ventriculoperitoneal shunt and was discharged on postoperative day 20 to acute inpatient rehabilitation in improved and stable condition. At the patient’s most recent follow-up (33 months after), there was the presence of a small area of enhancement at the anterior fossa, some ventricular enlargement, and mild enlargement of left parietal enhancing lesions on imaging. However, due to the patient’s clinical condition, it was felt that further treatment was not necessary. Clinically, the patient had improved physical, visual, and cognitive abilities. In addition, the patient demonstrated the ability to independently ambulate.
DISCUSSION
Within the context of intracranial tumors, metastatic LMS is rare, and primary intracranial LMS is rarely reported.[
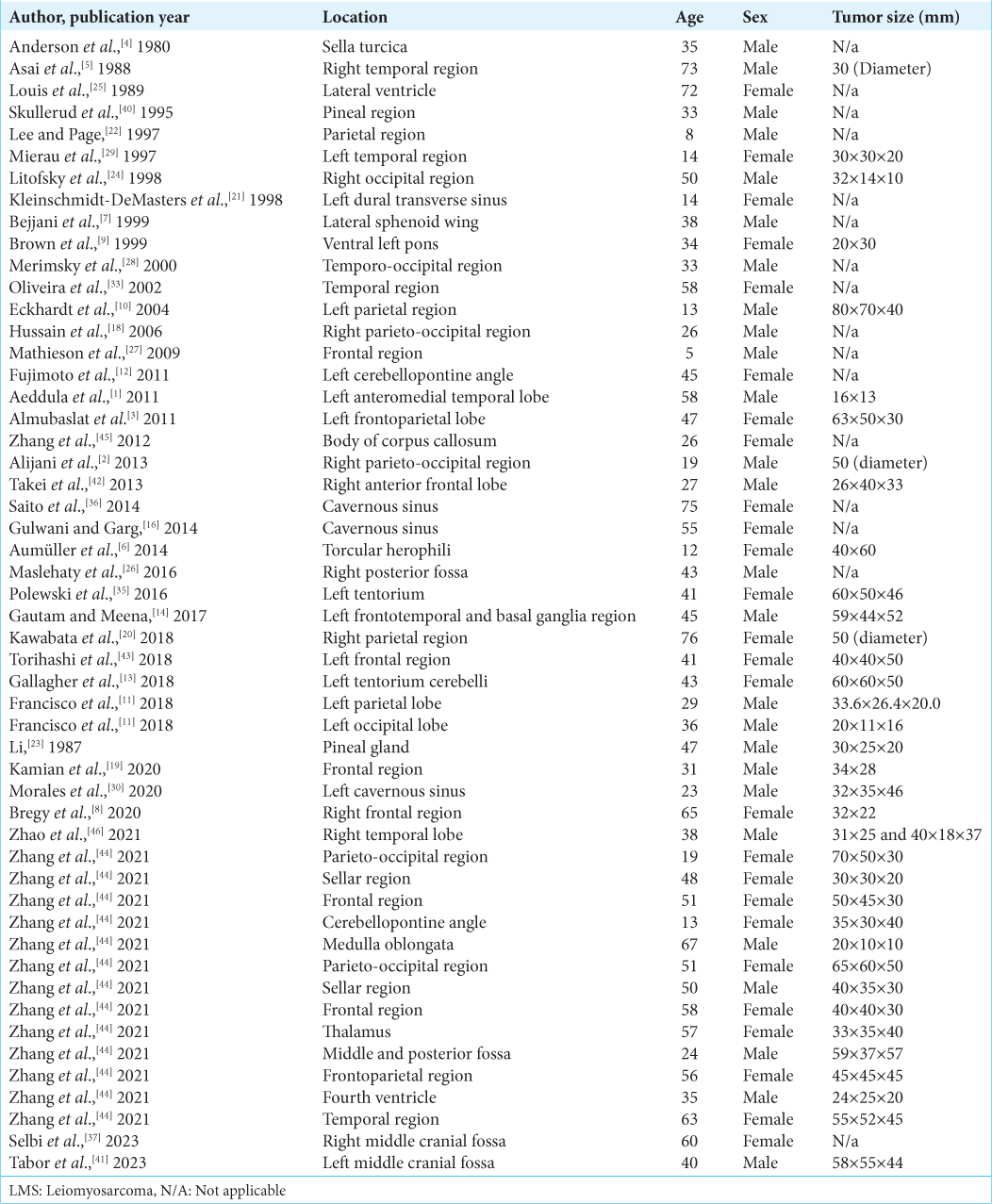
Examination of
While the histogenesis of intracranial LMS has not been fully elucidated, several theories pertaining to the origin of such tumors have been proposed. These theories are based on the idea that LMS tumors are mesenchymal-derived tumors. Therefore, conjectures have been proposed claiming transformation from the inner layers of the arachnoid, pia-mater,[
An alternative etiologic theory is vested in the association of LMS with HIV. Pang et al. demonstrated that mesenchymal stem cells play a role in macrophage, astrocyte, and T lymphocyte-mediated neuroinflammation.[
CONCLUSION
LMSs are rare tumors that seldom present in the CNS, therefore leading to a low index of suspicion in the preoperative differential diagnosis. When LMSs do occur, a history of immunocompromised state or previous radiation exposure is often present. Pathological confirmation is required for an appropriate diagnosis. Microsurgical resection of intracranial lesions when mass effect is present is highly recommended. Adjuvant chemotherapy and radiation appear to be effective treatment modalities; however, further research is necessary in CNS LMS.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We sincerely appreciate the artistic work of the figure by Jennifer Pryll.
References
1. Aeddula NR, Pathireddy S, Samaha T, Ukena T, Hosseinnezhad A. Primary intracranial leiomyosarcoma in an immunocompetent adult. J Clin Oncol. 2011. 29: e407-10
2. Alijani B, Yousefzade S, Aramnia A, Mesbah A. Primary intracranial leiomyosarcoma. Arch Iran Med. 2013. 16: 606-7
3. Almubaslat M, Stone JC, Liu L, Xiong Z. Primary intracranial leiomyosarcoma in an immunocompetent patient. Clin Neuropathol. 2011. 30: 154-7
4. Anderson WR, Cameron JD, Tsai SH. Primary intracranial leiomyosarcoma. Case report with ultrastructural study. J Neurosurg. 1980. 53: 401-5
5. Asai A, Yamada H, Murata S, Matsuno A, Tsutsumi K, Takemura T. Primary leiomyosarcoma of the dura mater. Case report. J Neurosurg. 1988. 68: 308-11
6. Aumüller M, Sykora KW, Hartmann C, Hermann EJ, Krauss JK. Primary intracranial leiomyosarcoma of the torcular Herophili associated with Fanconi anemia and allogenic stem cell transplantation. Childs Nerv Syst. 2014. 30: 1613-6
7. Bejjani GK, Stopak B, Schwartz A, Santi R. Primary dural leiomyosarcoma in a patient infected with human immunodeficiency virus: Case report. Neurosurgery. 1999. 44: 199-202
8. Bregy A, Lim J, Lohman R, Kane J, Prasad D, Qiu J. Primary leiomyosarcoma of the calvarium with intracranial extension: A case report. Indian J Surg Oncol. 2020. 11: 165-9
9. Brown HG, Burger PC, Olivi A, Sills AK, Barditch-Crovo PA, Lee RR. Intracranial leiomyosarcoma in a patient with AIDS. Neuroradiology. 1999. 41: 35-9
10. Eckhardt BP, Brandner S, Zollikofer CL, Wentz KU. Primary cerebral leiomyosarcoma in a child. Pediatr Radiol. 2004. 34: 495-8
11. Francisco CN, Alejandria M, Salvaña EM, Andal VM. Primary intracranial leiomyosarcoma among patients with AIDS in the era of new chemotherapeutic and biological agents. BMJ Case Rep. 2018. 2018: bcr2018225714
12. Fujimoto Y, Hirato J, Wakayama A, Yoshimine T. Primary intracranial leiomyosarcoma in an immunocompetent patient: Case report. J Neurooncol. 2011. 103: 785-90
13. Gallagher SJ, Rosenberg SA, Francis D, Salamat S, Howard SP, Kimple RJ. Primary intracranial leiomyosarcoma in an immunocompetent patient: Case report and review of the literature. Clin Neurol Neurosurg. 2018. 165: 76-80
14. Gautam S, Meena RK. Primary intracranial leiomyosarcoma presenting with massive peritumoral edema and mass effect: Case report and literature review. Surg Neurol Int. 2017. 8: 278
15. George S, Serrano C, Hensley ML, Ray-Coquard I. Soft tissue and uterine leiomyosarcoma. J Clin Oncol. 2018. 36: 144-50
16. Gulwani HV, Garg N. Primary extradural leiomyosarcoma involving cavernous sinus in an immunocompetent patient. Indian J Neurosurg. 2014. 3: 115-7
17. Hassanshahi G, Roohi MA, Esmaeili SA, Pourghadamyari H, Nosratabadi R. Involvement of various chemokine/chemokine receptor axes in trafficking and oriented locomotion of mesenchymal stem cells in multiple sclerosis patients. Cytokine. 2021. 148: 155706
18. Hussain S, Nanda A, Fowler M, Ampil FL, Burton GV. Primary intracranial leiomyosarcoma: Report of a case and review of the literature. Sarcoma. 2006. 2006: 52140
19. Kamian S, Ebrahimi A, Zadeh KE, Behzadi B. Primary intracranial leiomyosarcoma presenting with frontal bone mass: A case report. Radiat Oncol J. 2020. 38: 282-6
20. Kawabata Y, Aoki T, Yamamoto T, Yasui H, Sawai S, Fukuda S. Pazopanib-mediated long-term disease stabilization after local recurrence and distant metastasis of primary intracranial leiomyosarcoma: A case report on the efficacy of pazopanib as a salvage therapy. NMC Case Rep J. 2018. 5: 1-7
21. Kleinschmidt-DeMasters BK, Mierau GW, Sze CI, Breeze RE, Greffe B, Lillehei KO. Unusual dural and skull-based mesenchymal neoplasms: A report of four cases. Hum Pathol. 1998. 29: 240-5
22. Lee TT, Page LK. Primary cerebral leiomyosarcoma. Clin Neurol Neurosurg. 1997. 99: 210-2
23. Li NY. Primary leiomyosarcoma of the pineal gland--a case report. Zhonghua Zhong Liu Za Zhi. 1987. 9: 463-4
24. Litofsky NS, Pihan G, Corvi F, Smith TW. Intracranial leiomyosarcoma: A neuro-oncological consequence of acquired immunodeficiency syndrome. J Neurooncol. 1998. 40: 179-83
25. Louis DN, Richardson EP, Dickersin GR, Petrucci DA, Rosenberg AE, Ojemann RG. Primary intracranial leiomyosarcoma. Case report. J Neurosurg. 1989. 71: 279-82
26. Maslehaty H, Nabavi A, Mehdorn HM. Primary intracranial leiomyosarcoma: Case report and principles for treatment. J Neurol Neuromed. 2016. 1: 16-20
27. Mathieson CS, St George EJ, Stewart W, Sastry J, Jamal S. Primary intracranial leiomyosarcoma: A case report and review of the literature. Childs Nerv Syst. 2009. 25: 1013-7
28. Merimsky O, Lepechoux C, Terrier P, Vanel D, Delord JP, LeCesne A. Primary sarcomas of the central nervous system. Oncology. 2000. 58: 210-4
29. Mierau GW, Greffe BS, Weeks DA. Primary leiomyosarcoma of brain in an adolescent with common variable immunodeficiency syndrome. Ultrastruct Pathol. 1997. 21: 301-5
30. Morales RL, Álvarez A, Noreña MP, Torres F, Esguerra J. Low-grade leiomyosarcoma of the cavernous sinus in an HIV positive patient: Case report. Cureus. 2020. 12: e6758
31. Murakami T, Yamamoto N. Role of CXCR4 in HIV infection and its potential as a therapeutic target. Future Microbiol. 2010. 5: 1025-39
32. Nitzsche F, Müller C, Lukomska B, Jolkkonen J, Deten A, Boltze J. Concise review: MSC adhesion cascade-insights into homing and transendothelial migration. Stem Cells. 2017. 35: 1446-60
33. Oliveira AM, Scheithauer BW, Salomao DR, Parisi JE, Burger PC, Nascimento AG. Primary sarcomas of the brain and spinal cord: A study of 18 cases. Am J Surg Pathol. 2002. 26: 1056-63
34. Pang QM, Chen SY, Fu SP, Zhou H, Zhang Q, Ao J. Regulatory role of mesenchymal stem cells on secondary inflammation in spinal cord injury. J Inflamm Res. 2022. 15: 573-93
35. Polewski PJ, Smith AL, Conway PD, Marinier DE. Primary CNS leiomyosarcoma in an immunocompetent patient. J Oncol Pract. 2016. 12: 827-9
36. Saito N, Aoki K, Hirai N, Ishii M, Fujita S, Hiramoto Y. Primary intracranial leiomyosarcoma of the cavernous sinus: Case report. Austin J Med Oncol. 2014. 1: 2
37. Selbi W, Sims-Williams H, Ince P, Carroll TA. Skull base angiomatous leiomyoma: A case report and review of literature. Br J Neurosurg. 2023. 37: 668-70
38. Serrano C, George S. Leiomyosarcoma. Hematol Oncol Clin North Am. 2013. 27: 957-74
39. Sivendran S, Vidal CI, Barginear MF. Primary intracranial leiomyosarcoma in an HIV-infected patient. Int J Clin Oncol. 2011. 16: 63-6
40. Skullerud K, Stenwig AE, Brandtzaeg P, Nesland JM, Kerty E, Langmoen I. Intracranial primary leiomyosarcoma arising in a teratoma of the pineal area. Clin Neuropathol. 1995. 14: 245-8
41. Tabor JK, Lei H, Morales-Valero SF, O’Brien J, Gopal PP, Erson-Omay EZ. Epstein-Barr virus-associated primary intracranial leiomyosarcoma in an immunocompetent patient: Illustrative case. J Neurosurg Case Lessons. 2023. 5: CASE22532
42. Takei H, Powell S, Rivera A. Concurrent occurrence of primary intracranial Epstein-Barr virus-associated leiomyosarcoma and Hodgkin lymphoma in a young adult. J Neurosurg. 2013. 119: 499-503
43. Torihashi K, Chin M, Yoshida K, Narumi O, Yamagata S. Primary intracranial leiomyosarcoma with intratumoral hemorrhage: Case report and review of literature. World Neurosurg. 2018. 116: 169-73
44. Zhang GJ, Weng JC, Huo XL, Ma JP, Wang B, Wang L. Surgical management and long-term outcomes of primary intracranial leiomyosarcoma: A case series and review of literature. Neurosurg Rev. 2021. 44: 2319-28
45. Zhang H, Dong L, Huang Y, Zhang B, Ma H, Zhou Y. Primary intracranial leiomyosarcoma: Review of the literature and presentation of a case. Onkologie. 2012. 35: 609-16
46. Zhao L, Jiang Y, Wang Y, Bai Y, Sun Y, Li Y. Primary intracranial leiomyosarcoma secondary to glioblastoma: Case report and literature review. Front Oncol. 2021. 11: 642683