Pediatric Neurosurgery
Original Article
- Department of Neurosurgery, Fukuoka Children’s Hospital, Fukuoka, Japan
- Department of Neurosurgery, Hachisuga Hospital, Fukuoka, Japan
- Department of Psychiatry, Shourai Hospital, Karatsu, Japan
- Department of Neurosurgery, Kyushu University, Fukuoka, Japan
Correspondence Address:
Nobuya Murakami, Department of Neurosurgery, Fukuoka Children’s Hospital, Fukuoka, Japan.
DOI:10.25259/SNI_458_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nobuya Murakami1, Takato Morioka2, Ai Kurogi1, Satoshi O. Suzuki3, Takafumi Shimogawa4, Nobutaka Mukae4, Koji Yoshimoto4. Glial fibrillary acidic protein immunopositive neuroglial tissues with or without ependyma-lined canal in spinal lipoma of filar type: Relationship with retained medullary cord. 13-Sep-2024;15:326
How to cite this URL: Nobuya Murakami1, Takato Morioka2, Ai Kurogi1, Satoshi O. Suzuki3, Takafumi Shimogawa4, Nobutaka Mukae4, Koji Yoshimoto4. Glial fibrillary acidic protein immunopositive neuroglial tissues with or without ependyma-lined canal in spinal lipoma of filar type: Relationship with retained medullary cord. 13-Sep-2024;15:326. Available from: https://surgicalneurologyint.com/surgicalint-articles/glial-fibrillary-acidic-protein-immunopositive-neuroglial-tissues-with-or-without-ependyma-lined-canal-in-spinal-lipoma-of-filar-type-relationship-with-retained-medullary-cord/
Abstract
Background: Retained medullary cord (RMC) and filar lipomas are believed to originate from secondary neurulation failure; filar lipomas are reported to histopathologically contain a central canal-like ependyma-lined lumen with surrounding neuroglial tissue with ependyma-lined central canal (NGT w/E-LC) as a remnant of the medullary cord, which is a characteristic histopathology of RMC. With the addition of glial fibrillary acidic protein (GFAP) immunostaining, we reported the presence of GFAP-positive NGT without E-LCs (NGT w/o E-LCs) in RMC and filar lipomas, and we believe that both have the same embryopathological significance.
Methods: We examined the frequency of GFAP-positive NGT, with or without E-LC, in 91 patients with filar lipoma.
Results: Eight patients (8.8%) had NGT w/E-LC, 25 patients (27.5%) had NGT w/o E-LC, and 18 patients (19.8%) had tiny NGT w/o E-LC that could only be identified by GFAP immunostaining. Combining these subgroups, 56% of the patients (n = 51) with filar lipoma had GFAP immunopositive NGT.
Conclusion: The fact that more than half of filar lipomas have NGT provides further evidence that filar lipoma and RMC can be considered consequences of a continuum of regression failure that occurs during late secondary neurulation.
Keywords: Closed neural tube defect, Secondary neurulation, Spinal dysraphism, Cord tethering, Embryopathology
INTRODUCTION
Retained medullary cord (RMC) is an entity of closed spinal dysraphism that is believed to originate from secondary neurulation failure.[
NGT w/E-LC has been reported to be present in the filum. [
In contrast, solitary NGT without E-LC (NGT w/o E-LC) has been found in RMC and terminal lipoma;[
MATERIALS AND METHODS
A retrospective analysis was performed on 91 patients with filar lipomas (43 boys and 48 girls) who underwent initial untethering surgery at Fukuoka Children’s Hospital between September 2017 and February 2024. The median age at surgery was 2.9 years (range, 4 months to 20 years). Patients with spinal dysraphism attributed to first neurulation failure were excluded from the study. A diagnosis of filar lipoma was made from the findings of preoperative magnetic resonance imaging based and Arai’s classification.[
Five patients with symptoms, such as leg pain or mild motor weakness, underwent untethering surgery. Before spinal fusion and correction surgery, 29 patients with scoliosis underwent preventive surgery. Sixteen patients with low-lying conus (below L2/3) underwent preventive surgery. Other patients underwent preventive surgery after discussions with their parents.
In principle, minimally invasive surgery is performed through preexisting spina bifida or single-level laminoplastic laminotomy at L5 or S1. Intraoperatively, the fatty filum was segregated using a rubber dam and stimulated with a current intensity of up to 3 mA to confirm that it was non-functional. The filum was resected as a column. Surgical specimens were placed in formalin and performed with H&E staining and immunostaining for GFAP.
The clinical and neuroradiological findings have been analyzed as in previous clinical studies on filar lipomas.[
RESULTS
Eight patients (8.8% of the total patients) had NGT w/E-LC [
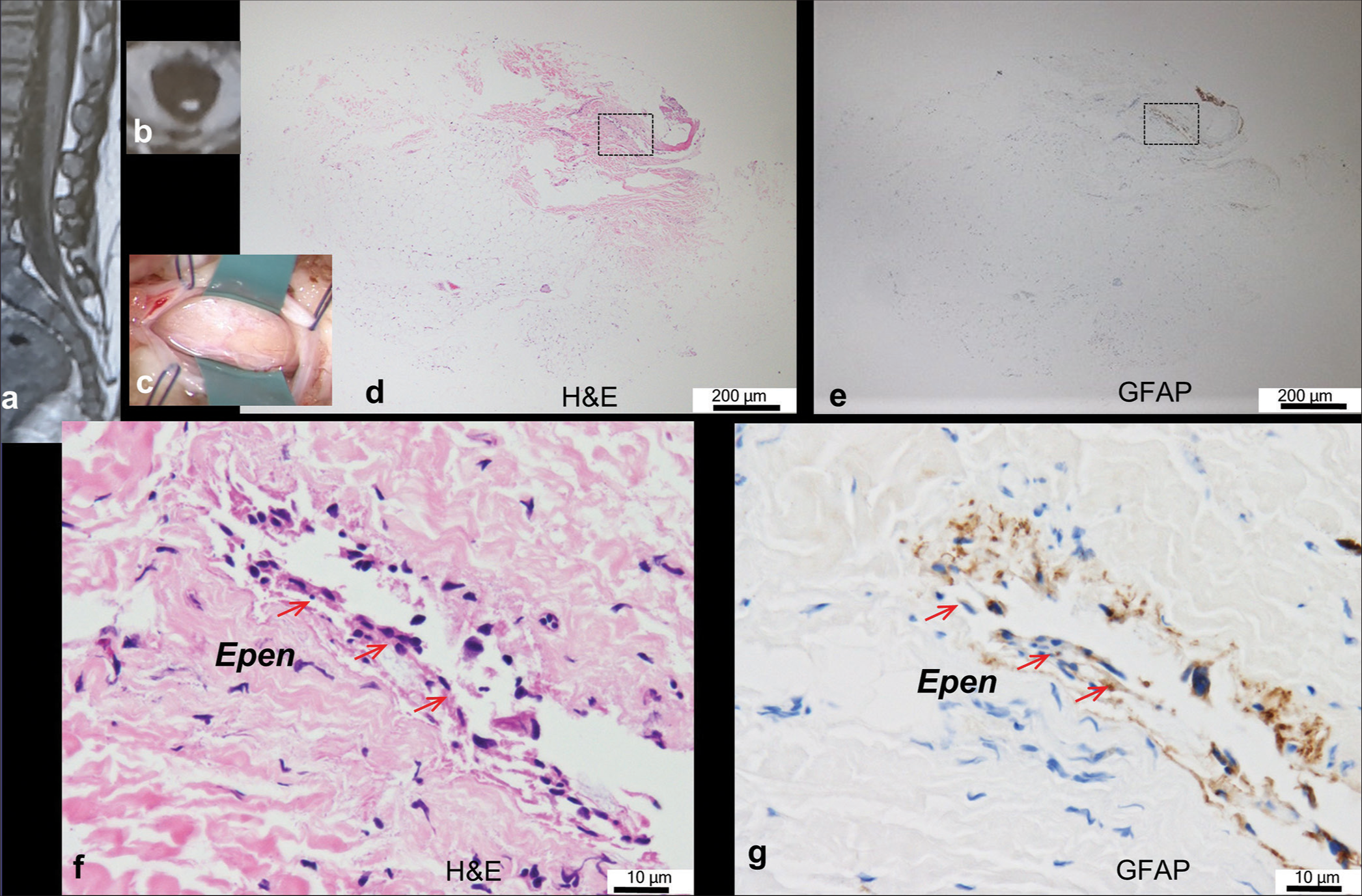
Figure 1:
Typical case of neuroglial tissue with ependyma-lined central canal (there are eight similar cases in total). (a, b) Sagittal and axial views of the T1-weighted image show a fatty filum with a low-lying spinal cord tapering to the dural cul-de-sac. (c) The intraoperative photograph shows the lipoma of filar type with a diameter of 4.0 mm through laminotomy at L5. (d-g) Histopathological findings of the transverse sections of the lipoma stained with hematoxylin and eosin (H&E; (d, f)) and immunostained with glial fibrillary acidic protein ((GFAP); (e, g)). Higher-magnification views of the area are indicated by dashed squares in (d) and (e). A single ependyma-lined canal (Epen; red arrows in (f) and (g)) with surrounding neuroglial tissues is embedded in the periphery of lipomatous fibroadipose tissue.
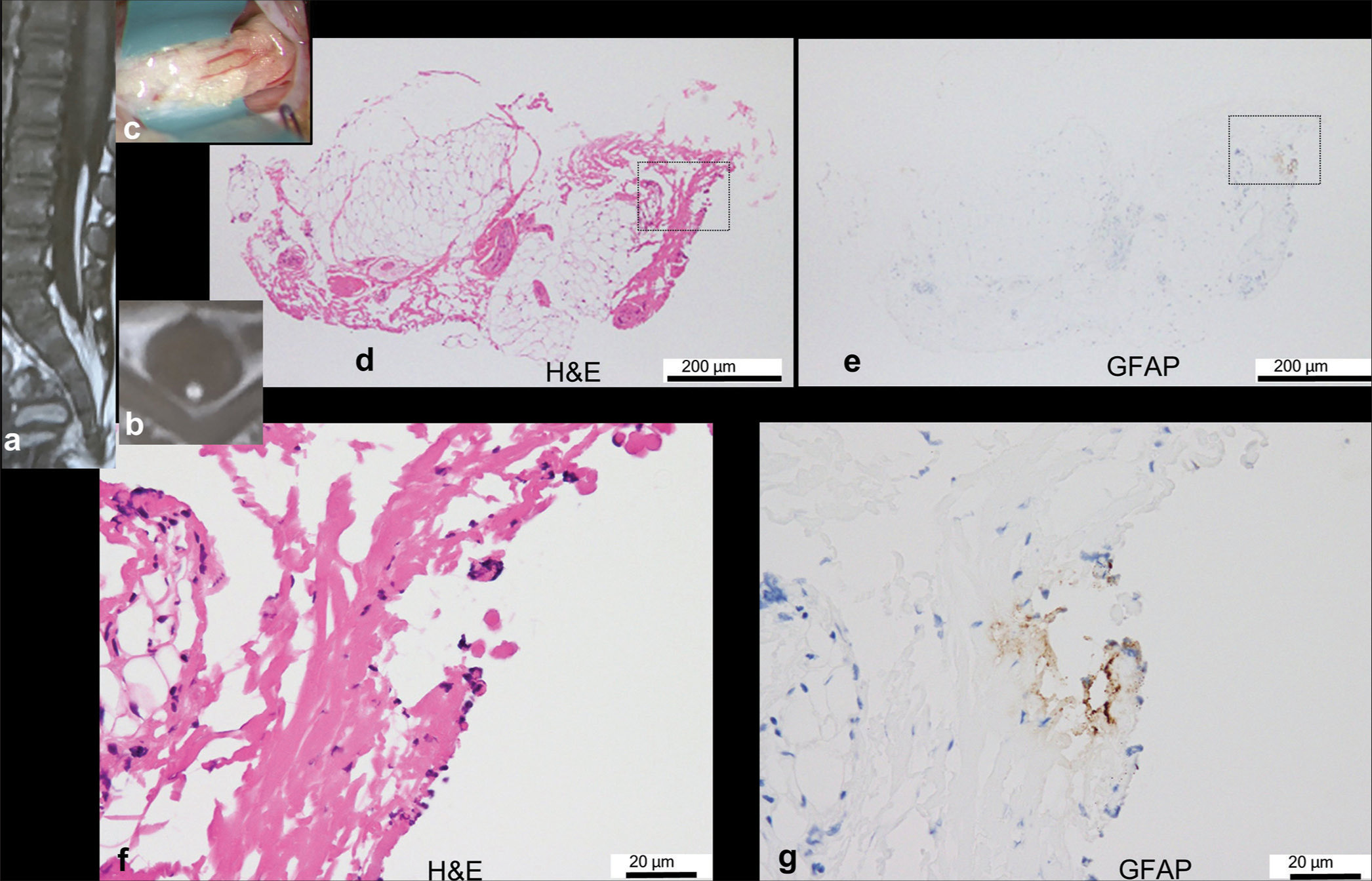
Figure 2:
Typical case of neuroglial tissue without ependyma-lined central canal (there are 25 similar cases in total). (a,b) Sagittal and axial views of the T1-weighted image show fatty filum. (c) Sagittal views of three-dimensional heavily T2-weighted imaging show a filar cyst (red arrow). (d) Intraoperative photograph showing the filar lipoma with a diameter of 4.0 mm through the laminotomy at L5. (e-j) Histopathological findings of the transverse sections of the lipoma stained with hematoxylin and eosin (H&E; e, g, i) and immunostained with glial fibrillary acidic protein (GFAP; f, h, j). Higher-magnification views of the area are indicated by dashed squares in (e) and (f). Neuroglial tissues are focally attached to fibroadipose tissue, which is clearly delineated by GFAP but barely by H&E. There is no central canal lined by ependymal cells.
Figure 3:
Typical case of tiny neuroglial tissue (there are 18 similar cases in total). (a, b) Sagittal and axial views of the T1-weighted image show fatty filum with lower-lying conus. (c) Intraoperative photograph showing filar lipoma with a diameter of 1.5 mm through laminotomy at S1. (d-g) Histopathological findings of the transverse sections of the lipoma stained with hematoxylin and eosin (H&E; d, f) and immunostained with glial fibrillary acidic protein (GFAP; e, g). Higher-magnification views of the area are indicated by dashed squares in (d) and (e). Tiny neuroglial tissues are attached to the periphery of fibroadipose tissue, which can only be detected by GFAP but not by H&E. There is no central canal lined with ependymal cells.
We compared the clinical features of patients with and without NGT, including age at surgery, syndromic status, preoperative symptoms, cutaneous signs, and surgery for scoliosis. The age at surgery of patients with NGT (median, 1.7 years; range, 4 months to 16 years) was significantly younger than those without NGT (median, 8.5 years; range, 6 months to 20 years). This was because the w/o NGT group included more patients before scoliosis surgery, who were all aged ≥6 years, than the w/NGT group. There were no significant differences in other items.
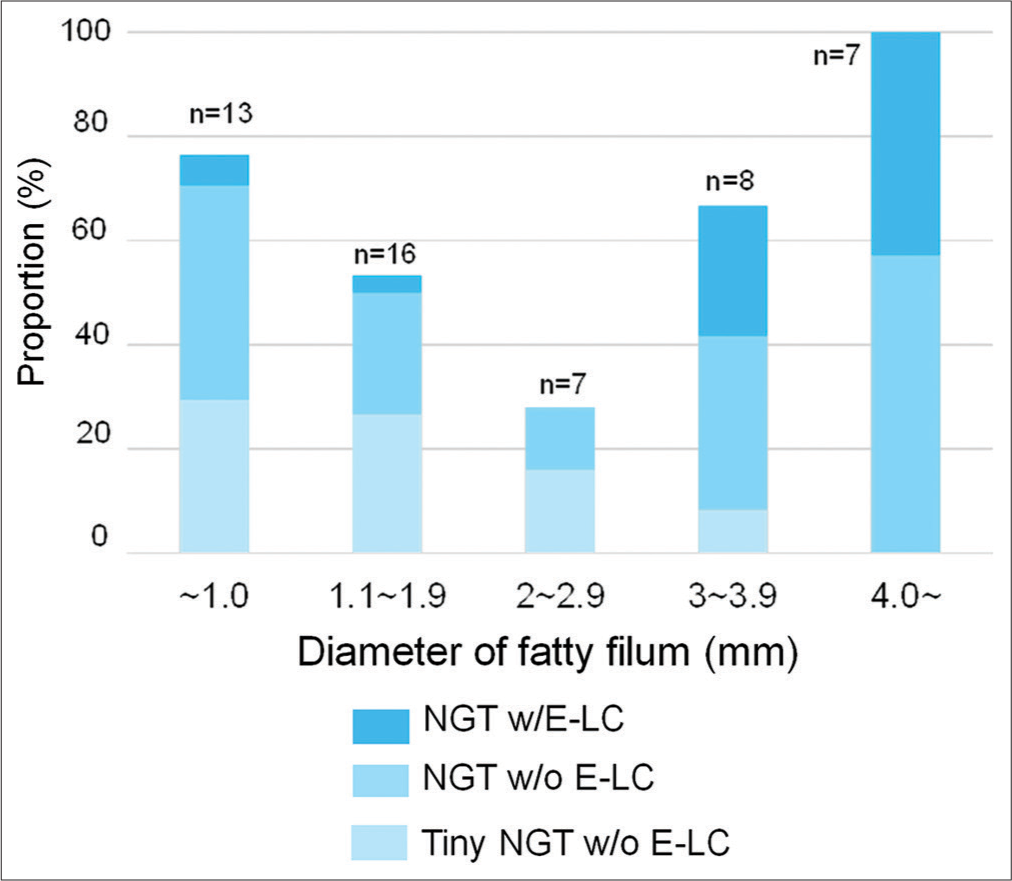
Morphological features, including conus level, diameter of the fatty filum, and association of filar cysts or syrinx, were also assessed between the w/and w/o NGT groups, and there were no significant differences in these parameters. From the perspective of the fatty filum diameter, an extremely thick filum (≥4.0 mm) was exclusively involved in the NGT w/ELC and NGT w/o E-LC groups, in which there was a certain amount of NGT, although other thinner filum was involved in various types [
DISCUSSION
The present study showed that NGT w/E-LC was found in 8.8% of 91 patients with filar lipoma, while in our previous report, E-LC w/NGT was observed in 4 (17.4%) out of 23 patients with filar lipoma.[
The most notable finding of the present study was that NGT w/o E-LC was observed frequently (47.3%). Adding the NGT w/E-LC to this, GFAP immunopositive NGT was observed in 56% of filar lipomas. The possible reason why most NGT lack E-LC is that small islands of E-LC may have been missed during routine sectioning of the surgical specimen, as previously described[
There were no significant differences in clinical background, except for age at operation, between the groups with and without NGT. The finding that NGT is absent more often in elderly patients may suggest age-dependent degeneration of the filum, although the detailed mechanism is unknown.
As we previously reported cases of large filar cysts associated with terminal lipomas, including filar and caudal types, which are histopathologically cystic dilatations of the E-LC with a surrounding NGT, indicating cystic RMC,[
The terminology for RMC and lipoma is based on their embryological and morphological background, respectively.[
This study has several limitations. First, as mentioned in the Introduction, it has been reported that normal filum also contains NGT[
CONCLUSION
Although further study to improve the above limitations will be needed, the 56% incidence of the association of GFAP immunopositive NGT with filar lipoma is believed to be due to secondary neurulation failure with the same embryological background as RMC. These results may provide a deeper understanding of the embryogenic background of lumbosacral spinal lipomas.
Ethical approval
The research/study approved by the Institutional Review Board at Fukuoka Children’s Hospital, number 2021-713, dated July 5, 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Arai H, Sato K, Okuda O, Miyajima M, Hishii M, Nakanishi H. Surgical experience of 120 patients with lumbosacral lipomas. Acta Neurochir (Wien). 2001. 143: 857-64
2. Choi BH, Kim RC, Suzuki M, Choe W. The ventriculus terminalis and filum terminale of the human spinal cord. Hum Pathol. 1992. 23: 916-20
3. Cools MJ, Al-Holou WN, Stetler WR, Wilson TJ, Muraszko KM, Ibrahim M. Filum terminale lipomas: Imaging prevalence, natural history, and conus position. J Neurosurg Pediatr. 2014. 13: 559-67
4. Durdağ E, Börcek PB, Öcal Ö, Börcek AÖ, Emmez H, Baykaner MK. Pathological evaluation of the filum terminale tissue after surgical excision. Childs Nerv Syst. 2015. 31: 759-63
5. Kim KH, Lee JY, Wang KC. Secondary neurulation defects-1: Retained medullary cord. J Korean Neurosurg Soc. 2020. 63: 314-20
6. Lellouch-Tubiana A, Zerah M, Catala M, Brousse N, Kahn AP. Congenital intraspinal lipomas: Histological analysis of 234 cases and review of the literature. Pediatr Dev Pathol. 1999. 2: 346-52
7. Morioka T, Murakami N, Kanata A, Tsukamoto H, Suzuki SO. Retained medullary cord with sacral subcutaneous meningocele and congenital dermal sinus. Childs Nerv Syst. 2020. 36: 423-7
8. Morioka T, Murakami N, Ichiyama M, Kusuda T, Suzuki SO. Congenital dermal sinus elements in each tethering stalk of coexisting thoracic limited dorsal myeloschisis and retained medullary cord. Pediatr Neurosurg. 2020. 55: 380-7
9. Morioka T, Murakami N, Suzuki SO, Mukae N, Shimogawa T, Kurogi A. Surgical histopathology of a filar anomaly as an additional tethering element associated with closed spinal dysraphism of primary neurulation failure. Surg Neurol Int. 2021. 12: 373
10. Morioka T, Murakami N, Kurogi A, Mukae N, Shimogawa T, Shono T. Embryopathological relationship between retained medullary cord and caudal spinal lipoma. Interdiscip Neurosurg. 2022. 29: 101534
11. Morota N, Ihara S, Ogiwara H. New classification of spinal lipomas based on embryonic stage. J Neurosurg Pediatr. 2017. 19: 428-39
12. Mukae N, Morioka T, Suzuki SO, Murakami N, Shimogawa T, Kanata A. Two Cases of large filar cyst associated with terminal lipoma: Relationship with retained medullary cord. World Neurosurg. 2020. 142: 294-8
13. Murakami N, Morioka T, Shimogawa T, Mukae N, Inoha S, Sasaguri T. Ependyma-lined canal with surrounding neuroglial tissues in lumbosacral lipomatous malformations: Relationship with retained medullary cord. Pediatr Neurosurg. 2018. 53: 387-394
14. Murakami N, Morioka T, Shimogawa T, Hashiguchi K, Mukae N, Uchihashi K. Retained medullary cord extending to a sacral subcutaneous meningocele. Childs Nerv Syst. 2018. 34: 527-33
15. Oketani H, Harimaya K, Ono T, Terado K, Inoha S, Suzuki SO. A presenile patient with filar lipoma who developed tethered spinal cord syndrome triggered by lumbar canal stenosis. NMC Case Rep J. 2023. 10: 109-13
16. Pang D, Zovickian J, Moes GS. Retained medullary cord in humans: Late arrest of secondary neurulation. Neurosurgery. 2011. 68: 1500-19
17. Pang D, Chong S, Wang KC, Di Rocco C, Pang D, Rutka JT, editors. Secondary neurulation defects-1: Thickened filum terminale, retained medullary cord. Textbook of pediatric neurosurgery. Switzerland: Springer; 2020. p.
18. Sala F, Barone G, Tramontano V, Gallo P, Ghimenton C. Retained medullary cord confirmed by intraoperative neurophysiological mapping. Childs Nerv Syst. 2014. 30: 1287-91
19. Selçuki M, Vatansever S, Inan S, Erdemli E, Baĝdatoĝlu C, Polat A. Is a filum terminale with a normal appearance really normal?. Childs Nerv Syst. 2003. 19: 3-10
20. Shirozu N, Morioka T, Inoha S, Imamoto N, Sasaguri T. Enlargement of sacral subcutaneous meningocele associated with retained medullary cord. Childs Nerv Syst. 2018. 34: 1785-90
21. Sim J, Shim Y, Kim KH, Kim SK, Lee JY. Features of the filum terminale in tethered cord syndrome with focus on pathology. J Korean Neurosurg Soc. 2021. 64: 585-91
22. Usami K, Lallemant P, Roujeau T, James S, Beccaria K, Levy R. Spinal lipoma of the filum terminale: Review of 174 consecutive patients. Childs Nerv Syst. 2016. 32: 1265-72
23. Walsh JW, Markesbery WR. Histological features of congenital lipomas of the lower spinal canal. J Neurosurg. 1980. 52: 564-9