- College of Medicine, Alfaisal University, Riyadh, Saudi Arabia
- Department of Surgery, Division of Otolaryngology - Head and Neck Surgery, University of Ottawa, Ottawa, Ontario, Canada
- Department of Neurosurgery, Barrow Neurological Institute and St. Joseph's Hospital and Medical Center, Phoenix, Arizona, USA
Correspondence Address:
Mohamed A. Labib
Department of Neurosurgery, Barrow Neurological Institute and St. Joseph's Hospital and Medical Center, Phoenix, Arizona, USA
DOI:10.4103/2152-7806.177888
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Abou Al-Shaar H, Macdonald KI, Labib MA. Glomangiopericytoma simulating an intracavernous meningioma. Surg Neurol Int 02-Mar-2016;7:
How to cite this URL: Abou Al-Shaar H, Macdonald KI, Labib MA. Glomangiopericytoma simulating an intracavernous meningioma. Surg Neurol Int 02-Mar-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/glomangiopericytoma-simulating-an-intracavernous-meningioma-2/
Abstract
Background:Glomangiopericytoma is an uncommonly encountered tumor of the nose and paranasal sinuses, accounting for
Case Description:We report a case of a giant glomangiopericytoma localizing into the cavernous sinus in a 48-year-old female who presented with mild left-sided ptosis for 48 months. The lesion simulated an intracavernous meningioma on preoperative imaging. An expanded endoscopic endonasal approach was used to debulk the portion of the lesion in the medial compartment of the cavernous sinus. Postoperatively, the patient's ptosis resolved completely, and no new deficits were sustained.
Conclusion:This is the only case of glomangiopericytoma localizing solely to the cavernous sinus reported to date.
Keywords: Cavernous sinus, endoscopic approach, glomangiopericytoma, meningioma, sinonasal-type hemangiopericytoma
INTRODUCTION
Glomangiopericytoma is an extremely rare tumor of the nose and paranasal sinuses, accounting for <0.5% of all sinonasal tumors.[
CASE REPORT
History and examination
A 48-year-old female presented with a very mild left sided ptosis for 48 months. She denied any symptoms suggestive of increased intracranial pressure or endocrinological abnormalities. Her past medical and family histories were noncontributory.
Physical examination revealed a very mild left sided ptosis without any ophthalmoplegia. The remainder of her examination was unremarkable.
Imaging
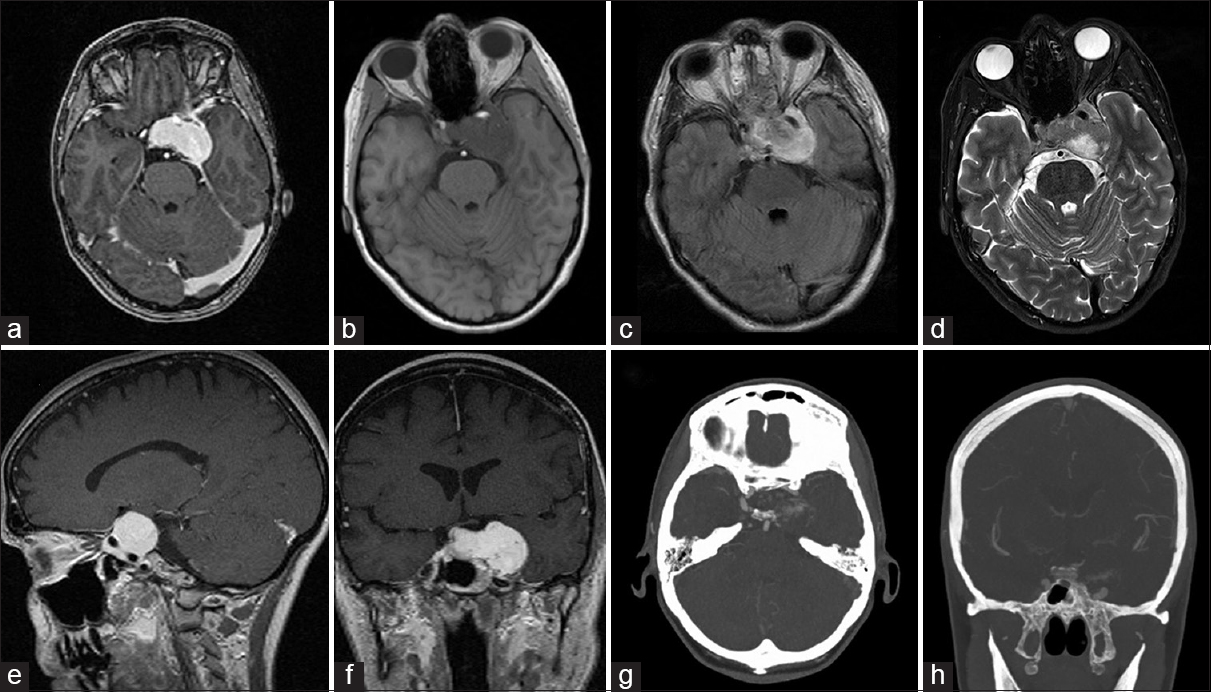
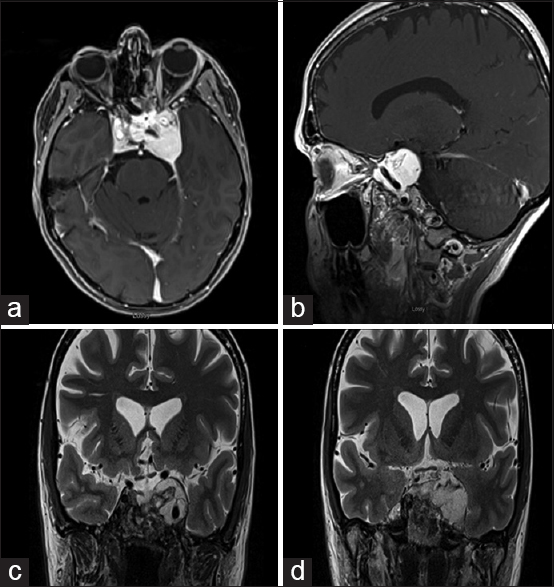
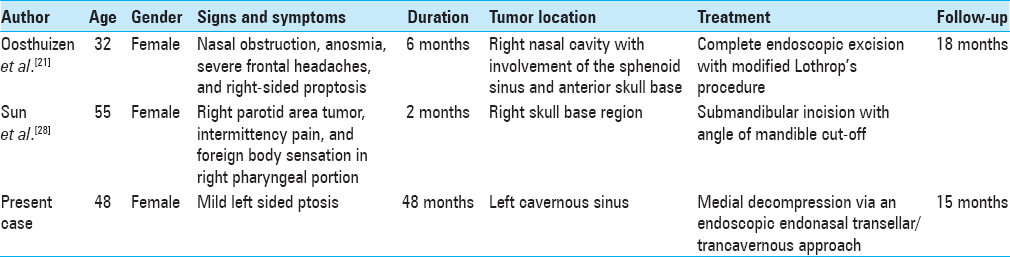
Imaging which included a computed tomography (CT) scan and a magnetic resonance imaging (MRI) revealed a large sellar/parasellar/intracavernous lesion that avidly enhanced. The lesion displaced the pituitary gland laterally to the right side as well as the left parasellar internal carotid artery inferiorly [
Figure 1
Preoperative imaging of the glomangiopericytoma. Axial T1 magnetic resonance imaging with gadolinium (a), T1 without gadolinium (b), flair (c), T2 (d), sagittal (e), and coronal (f) T1 with gadolinium depicting the tumor in the left cavernous sinus simulating an intracavernous meningioma. Axial (g) and coronal (h) computed tomography angiography demonstrating low vascular flow to the tumor in the left cavernous sinus
Surgical management
To obtain tissue diagnosis and decompress the medial compartment of the cavernous sinus, an endoscopic endonasal transellar/trancavernous approach was undertaken. Intraoperatively, the lesion proved to be extremely hemorrhagic. Eight units of blood were transfused intraoperatively. Medial decompression of the cavernous sinus was successfully accomplished.
Histopathological findings
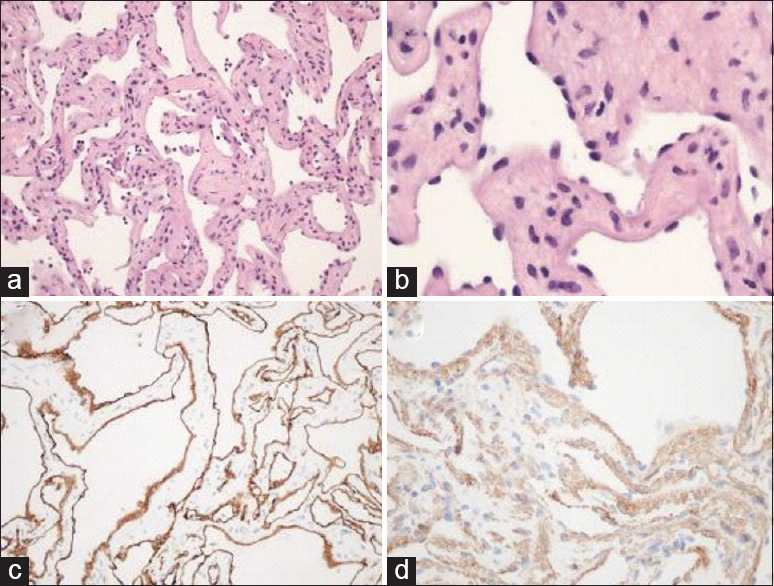
The lesional tissue showed connective vascular arrangements in a kind of honeycomb structure, with a relatively thin walls consisting of endothelial cells and fibroblast-like cells in the stroma. Mitotic figures and necrosis were not seen. In the vascular spaces neutrophils were found, but hardly erythrocytes. The vascular walls did not contain elastin, or collagen bundles. On immunohistochemical studies, the specimen was negative for CD34 and positive for smooth muscle markers (smooth muscle actin [SMA], muscle specific actin [MSA], and desmin) and vimentin in the stroma only. CD34, ETS-related gene (ERG), and factor VIII were strongly positive in the endothelium. Based on these findings a diagnosis of glomangiopericytoma was established [
Figure 2
Microscopic and immunohistochemical examination of the resected specimen (a and b). Low and high power microscopic examination with H and E stain demonstrating connective vascular arrangements in a kind of honeycomb structure, with a relatively thin walls consisting of endothelial cells and fibroblast-like cells in the stroma. Immunohistochemical examination of the specimen showed diffuse and strong positivity to CD34 in the endothelium alone (c) and muscle-specific actin in the stroma (d)
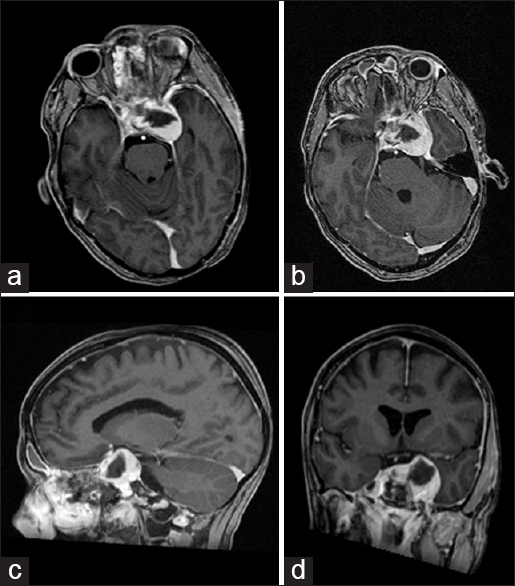
Postoperative course
Postoperatively, the patient's ptosis resolved completely. She did not develop any new neurological symptoms. Postoperative imaging confirmed successful medical decompression of the cavernous sinus [
DISCUSSION
Glomangiopericytomas are uncommonly encountered neoplasms of the sinonasal region with a characteristic perivascular myoid phenotype.[
The development of glomangiopericytomas within the sinonasal region remains elusive. Although many factors including trauma, hypertension, pregnancy, and long-term corticosteroids use have been proposed as the etiological agents for the development of these tumors, no common consensus have been reached regarding the association of these etiological factors and the development of glomangiopericytoma.[
Diagnosing glomangiopericytoma is challenging, as it is frequently confused with other sinonasal lesions. Thus, endoscopic evaluation and radiological imaging are essential to delineate the tumor location, extension, and guide surgical intervention. On MRI, these tumors appear isointense on T1-attentuated imaging and isointense-to-hypointense on T2-attenuated images.[
On gross examination, these tumors may appear as solid, firm, red or fleshy, soft, and hemorrhagic polypoid edematous masses of variable sizes (mean 3.1 cm; range 1–8 cm).[
The tumor cells are strongly positive for actin and vimentin on immunohistochemical staining.[
It is therefore important to include glomangiopericytoma in the differential diagnosis of spindle cell tumors of the sinonasal region, which also encompass solitary fibrous tumors, glomus tumors, lobular capillary hemangiomas, angiofibromas, and leiomyomas.[
Various treatment modalities have been reported for the management of glomangiopericytomas. Some authors advocate for conservative management in the form of tumor debulking and frequent endoscopic surveillance, especially when the tumor extends into the anterior skull base.[
With the recent advancements in the endoscopic techniques, some authors consider endoscopic surgery as the modality of choice for tumors involving the sinonasal and anterior skull base regions.[
As opposed to hemangiopericytomas, glomangiopericytoma generally behave in an indolent manner, with extremely low malignant and metastatic potentials.[
The estimated recurrence rate for glomangiopericytoma ranges from 7% to 40%; recurrence can be managed by additional surgery and/or adjuvant radiotherapy.[
The role of radiation therapy in the management of residual tumors or primary lesions not amenable to surgical resection is still controversial. Glomangiopericytomas are generally radiation resistant tumors.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abraham SC, Montgomery EA, Giardiello FM, Wu TT. Frequent beta-catenin mutations in juvenile nasopharyngeal angiofibromas. Am J Pathol. 2001. 158: 1073-8
2. Angouridakis N, Zaraboukas T, Vital J, Vital V. Sinonasal hemangiopericytoma of the middle turbinate: A case report and brief review of the literature. B-ENT. 2007. 3: 139-43
3. Arpaci RB, Kara T, Vayisoglu Y, Ozgur A, Ozcan C. Sinonasal glomangiopericytoma. J Craniofac Surg. 2012. 23: 1194-6
4. Barnes L, Eveson JW, Reichart P, Sidransky DPathology and Genetics of Head and Neck Tumours. World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2005. 9: 43-4
5. Bhattacharyya N, Shapiro NL, Metson R. Endoscopic resection of a recurrent sinonasal hemangiopericytoma. Am J Otolaryngol. 1997. 18: 341-4
6. Bignami M, Dallan I, Battaglia P, Lenzi R, Pistochini A, Castelnuovo P. Endoscopic, endonasal management of sinonasal haemangiopericytoma: 12-year experience. J Laryngol Otol. 2010. 124: 1178-82
7. Birzgalis AR, Ramsden RT, Lye RH, Richardson PL. Haemangiopericytoma of the temporal bone. J Laryngol Otol. 1990. 104: 998-1003
8. Brandwein-Gensler M, Siegal GP. Striking pathology gold: A singular experience with daily reverberations: Sinonasal hemangiopericytoma (glomangiopericytoma) and oncogenic osteomalacia. Head Neck Pathol. 2012. 6: 64-74
9. Compagno J. Hemangiopericytoma-like tumors of the nasal cavity: A comparison with hemangiopericytoma of soft tissues. Laryngoscope. 1978. 88: 460-9
10. Dandekar M, McHugh JB. Sinonasal glomangiopericytoma: Case report with emphasis on the differential diagnosis. Arch Pathol Lab Med. 2010. 134: 1444-9
11. Eichhorn JH, Dickersin GR, Bhan AK, Goodman ML. Sinonasal hemangiopericytoma. A reassessment with electron microscopy, immunohistochemistry, and long-term follow-up. Am J Surg Pathol. 1990. 14: 856-66
12. Gillman G, Pavlovich JB. Sinonasal hemangiopericytoma. Otolaryngol Head Neck Surg. 2004. 131: 1012-3
13. Hansen T, Katenkamp K, Katenkamp D. D2-40 staining in sinonasal-type hemangiopericytoma - Further evidence of distinction from conventional hemangiopericytoma and solitary fibrous tumor. Virchows Arch. 2006. 448: 459-62
14. Hervé S, Abd Alsamad I, Beautru R, Gaston A, Bedbeder P, Peynègre R. Management of sinonasal hemangiopericytomas. Rhinology. 1999. 37: 153-8
15. Higashi K, Nakaya K, Watanabe M, Ikeda R, Suzuki T, Oshima T. Glomangiopericytoma of the nasal cavity. Auris Nasus Larynx. 2011. 38: 415-7
16. Kuo FY, Lin HC, Eng HL, Huang CC. Sinonasal hemangiopericytoma-like tumor with true pericytic myoid differentiation: A clinicopathologic and immunohistochemical study of five cases. Head Neck. 2005. 27: 124-9
17. Leong JL, Citardi MJ, Batra PS. Reconstruction of skull base defects after minimally invasive endoscopic resection of anterior skull base neoplasms. Am J Rhinol. 2006. 20: 476-82
18. Montag AG, Tretiakova M, Richardson M. Steroid hormone receptor expression in nasopharyngeal angiofibromas. Consistent expression of estrogen receptor beta. Am J Clin Pathol. 2006. 125: 832-7
19. Morrison EJ, Wei BP, Fancourt T, Lyons B. Glomangiopericytoma: Overview and role for open surgery. ANZ J Surg. 2012. 82: 648-50
20. Mosesson RE, Som PM. The radiographic evaluation of sinonasal tumors: An overview. Otolaryngol Clin North Am. 1995. 28: 1097-115
21. Oosthuizen JC, Kennedy S, Timon C. Glomangiopericytoma (sinonasal-type haemangiopericytoma). J Laryngol Otol. 2012. 126: 1069-72
22. Palacios E, Restrepo S, Mastrogiovanni L, Lorusso GD, Rojas R. Sinonasal hemangiopericytomas: Clinicopathologic and imaging findings. Ear Nose Throat J. 2005. 84: 99-102
23. Park MS, Araujo DM. New insights into the hemangiopericytoma/solitary fibrous tumor spectrum of tumors. Curr Opin Oncol. 2009. 21: 327-31
24. Reiner SA, Siegel GJ, Clark KF, Min KW. Hemangiopericytoma of the nasal cavity. Rhinology. 1990. 28: 129-36
25. Schlosser RJ, Woodworth BA, Gillespie MB, Day TA. Endoscopic resection of sinonasal hemangiomas and hemangiopericytomas. ORL J Otorhinolaryngol Relat Spec. 2006. 68: 69-72
26. Serrano E, Coste A, Percodani J, Hervé S, Brugel L. Endoscopic sinus surgery for sinonasal haemangiopericytomas. J Laryngol Otol. 2002. 116: 951-4
27. Snyderman CH, Carrau RL, Kassam AB, Zanation A, Prevedello D, Gardner P. Endoscopic skull base surgery: Principles of endonasal oncological surgery. J Surg Oncol. 2008. 97: 658-64
28. Sun Q, Zhang C, Chen W, He Y. The molecular mechanisms on glomangiopericytoma invasion. Orphanet J Rare Dis. 2013. 8: 152-
29. Thiringer JK, Costantino PD, Houston G. Sinonasal hemangiopericytoma: Case report and literature review. Skull Base Surg. 1995. 5: 185-90
30. Thompson LD, Miettinen M, Wenig BM. Sinonasal-type hemangiopericytoma: A clinicopathologic and immunophenotypic analysis of 104 cases showing perivascular myoid differentiation. Am J Surg Pathol. 2003. 27: 737-49
31. Thompson LD, Goldblum JR. Foundations in diagnostic pathology. Head and Neck Pathology. New York: Elsevier; 2006. p. 135-40
32. Thompson LD. Sinonasal tract glomangiopericytoma (hemangiopericytoma). Ear Nose Throat J. 2004. 83: 807-
33. Tse LL, Chan JK. Sinonasal haemangiopericytoma-like tumour: A sinonasal glomus tumour or a haemangiopericytoma?. Histopathology. 2002. 40: 510-7
34. Watanabe K, Saito A, Suzuki M, Yamanobe S, Suzuki T. True hemangiopericytoma of the nasal cavity. Arch Pathol Lab Med. 2001. 125: 686-90
35. Worden B, Getz A, Luo R, Hwang PH. Pathology quiz case 1.Glomangiopericytoma (sinonasal-type hemangiopericytoma [HPC]). Arch Otolaryngol Head Neck Surg. 2009. 135: 520-522