- Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
- Department of Neurology, International University of Health and Welfare Narita Hospital, Narita, Japan
- Division of Medical Technology, Department of Health Sciences, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
- Department of Clinical Chemistry and Laboratory Medicine, Kyushu University Hospital, Fukuoka, Japan.
- Department of Neurosurgery, Harasanshin Hospital, Fukuoka, Japan.
Correspondence Address:
Nobutaka Mukae, Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
DOI:10.25259/SNI_152_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ayumi Yonamoto1, Nobutaka Mukae1, Takafumi Shimogawa1, Taira Uehara2, Hioshi Shigeto3, Ayumi Sakata4, Masahiro Mizoguchi1, Koji Yoshimoto1, Takato Morioka5. Good seizure outcome after focal resection surgery for super-refractory status epilepticus: Report of two cases. 22-Apr-2022;13:164
How to cite this URL: Ayumi Yonamoto1, Nobutaka Mukae1, Takafumi Shimogawa1, Taira Uehara2, Hioshi Shigeto3, Ayumi Sakata4, Masahiro Mizoguchi1, Koji Yoshimoto1, Takato Morioka5. Good seizure outcome after focal resection surgery for super-refractory status epilepticus: Report of two cases. 22-Apr-2022;13:164. Available from: https://surgicalneurologyint.com/surgicalint-articles/11551/
Abstract
Background: There is scarce evidence regarding focal resection surgery for super-refractory status epilepticus (SRSE), which is resistant to general anesthetic treatment over 24 h. We report two patients with SRSE, in whom good seizure outcomes were obtained following focal resection surgery.
Case Description: Patient 1: A 58-year-old man who underwent left anterior temporal lobectomy with hippocampectomy at the age of 38 years after being diagnosed left medial temporal lobe epilepsy. After 19 years of surgery with no epileptic attacks, the patient developed SRSE. Electroencephalogram (EEG) demonstrated persistence of lateralized periodic discharges in the left frontotemporal region. On the 20th day after SRSE onset, resection of the frontal lobe and temporal lobe posterior to the resection cavity was performed. Patient 2: A 62-year-old man underwent craniotomy for anaplastic astrocytoma in the left frontal lobe at the age of 34 years. Since the age of 60 years, he developed SRSE 3 times over 1 and 1/12 years. On EEG, repeated ictal discharges were observed at the medial part of the left frontal region during the three SRSEs. Corresponding to the ictal EEG findings, high signals on diffusion-weighted magnetic resonance images and focal hypermetabolism on fluorodeoxyglucose-positron emission tomography were observed around the supplementary motor area, medial to the resection cavity. Resection surgery of the area was performed during the interictal period.
Conclusion: Good seizure outcome was obtained in the two cases which provide additional support for the recent concept of focal resection surgery as an indication for SRSE.
Keywords: Astrogliosis, Diffusion-weighted image, Electrocorticography, Electroencephalography, Positron emission tomography
INTRODUCTION
Status epilepticus (SE) is a condition in which one seizure lasts for 5 min or more, or two or more seizures occur consecutively without the patient regaining normal level of consciousness.[
SRSE is recognized as a neurological emergency disease that is difficult to treat and has a poor prognosis, with death occurring in 35% of cases and 13% suffering from serious neurological sequelae.[
CASE DESCRIPTION
Patient 1
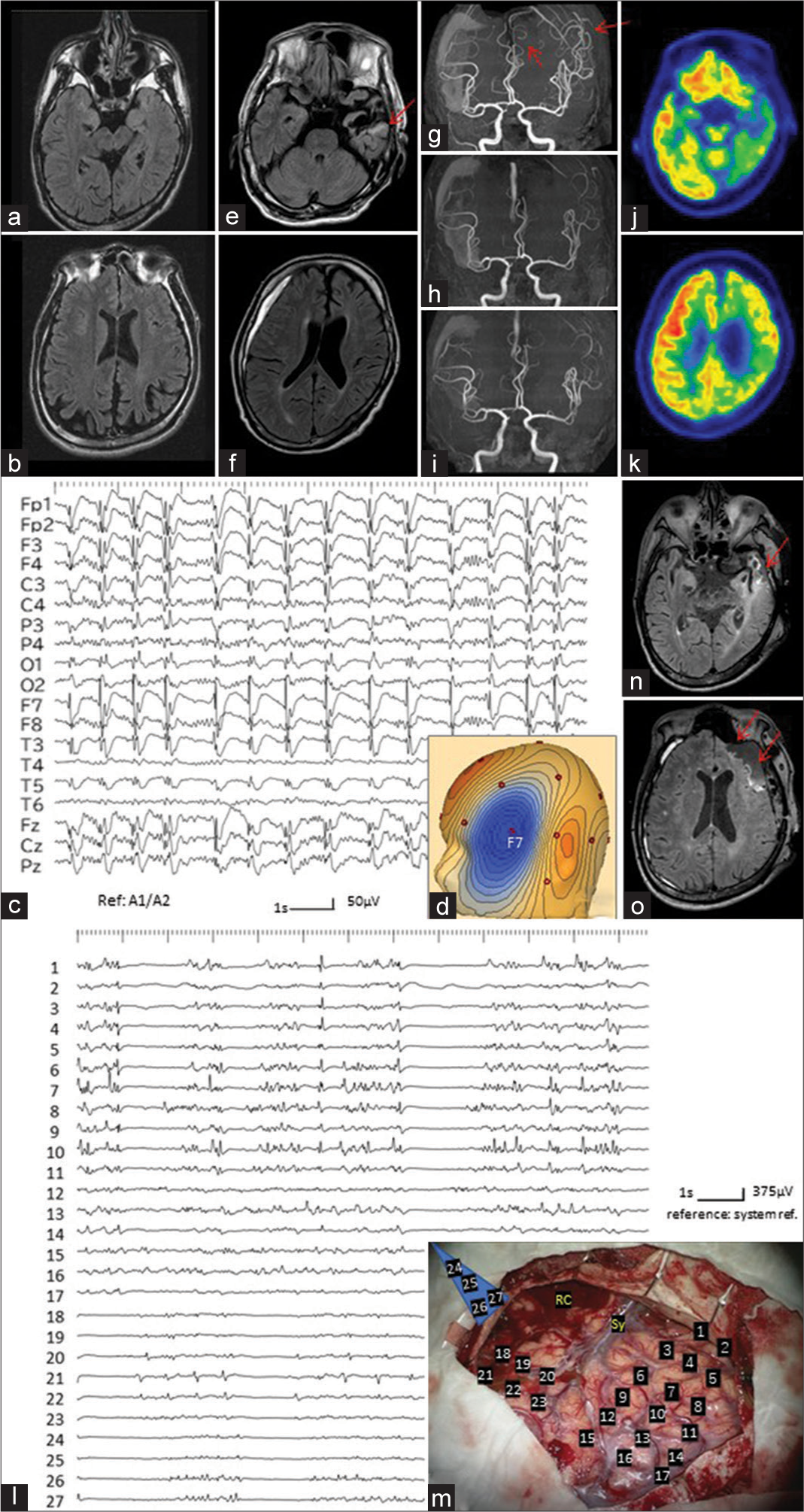
A 58-year-old man underwent anterior temporal lobectomy with hippocampectomy at the age of 38 years, with a diagnosis of left medial temporal lobe epilepsy (MTLE). In the Wada test performed as a preoperative examination at that time, it was determined that the right hemisphere was dominant in both language and memory. All clinical findings were typical of MTLE, and the findings of magnetic resonance (MR) images were characterized by a small left calvarium and left entire hemisphere, not just the left temporal lobe [
Figure 1:
(Patient 1) (a and b) Oblique views, along with the long axis of the hippocampus, of the magnetic resonance (MR) images with fluid attenuated inversion recovery (FLAIR) sequences, before the left anterior temporal lobectomy at the age of 38, demonstrate a small size of the left calvarium and left entire hemisphere, not just the left temporal lobe. (c) Electroencephalogram (EEG) on day 1 shows lateralized periodic discharges (LPD) at the left fronto-temporal region. (d) Voltage topography of LPD indicates that the maximum amplitude of LPD is located at F7 of International EEG 10–20 system. Blue indicates negativity. (e and f) Axial views of FLAIR images on day 2 depict gliosis in the temporal lobe posterior to the resection cavity (red arrow in e). No definite abnormality is noted at the left frontal lobe (f). Thin chronic subdural hematoma, caused by a fall due to generalized seizure, is also noted on the right side. (g) MR angiography shows increased signals of the peripheries of the left middle and anterior cerebral arteries due to “ictal hyperperfusion” (red arrows). (h and i) On MR angiography of day 9 (h) and day 17 (i), the ictal hyperperfusion is improved. (j and k) Positron emission tomography with fluorodeoxyglucose on day 19 shows rather low metabolism not just in the left temporal lobe, posterior to the resection cavity, but the left entire hemisphere. (l) Intraoperative electrocorticography depicts high-amplitude periodic discharges on the frontal lobe (electrode No 1-10). Asynchronous small-amplitude paroxysms are also recorded on the temporal lobe posterior to the resection cavity (electrode No. 18-22). No paroxysmal discharges are recorded from the medial part of the temporal lobe (electrode No. 24-27). (m) The location and number of the electrodes are indicated on the operative view. Blue trapezoid electrode (No. 24-27) is placed adjoining the medial and basal aspects of the temporal lobe, as described before.[11] Sy: Sylvian veins, RC: Resection cavity at the previous craniotomy. (n and o) Oblique views of FLAIR images, immediately after the operation, showing the resection area (red arrows).
However, at the age of 57, he had two generalized seizures, and AED administration, such as levetiracetam (LEV) and lacosamide (LCM), were resumed. Further, at the age of 58 years, he developed a third generalized seizure and was admitted because of SE (day 1). Intravenous injections of diazepam (DZP) and fosphenytoin (fPHT) resulted in cessation of the seizures, but impaired consciousness was subsequently prolonged. On the electroencephalogram (EEG), lateralized periodic discharges (LPD) with the maximum amplitude at F7 of the International EEG 10–20 system were recorded in the left frontotemporal region [
General anesthesia was maintained with the appearance of a suppression-burst on the EEG monitoring, and the ictal hyperperfusion demonstrated by MRA improved on day 9 [
Based on the EEG findings, we considered that there was an epileptogenic region at the left fronto-temporal lobe, and on day 20, the fronto-temporo-parietal craniotomy was performed under general total intravenous anesthesia (TIVA) with propofol. Intraoperative electrocorticography (ECoG) showed high-amplitude periodic discharges in the frontal lobe. Asynchronous small-amplitude paroxysms were also recorded in the temporal lobe posterior to the resection cavity [
After the operation, general anesthesia was discontinued under EEG monitoring for 2 days, and controlled ventilation was withdrawn. The patient was completely awake on the 10th postoperative day. Postoperative EEG occasionally showed low-amplitude paroxysmal discharges in the left frontotemporal region, but no seizures occurred. On the 15th postoperative day, the patient was transferred to a rehabilitation hospital with the prescription of LEV, LCM, PER, CLB, and phenytoin, and the tracheostomy was closed on the 43rd postoperative day. Pathological examination of the resected frontal and temporal lobes revealed marked astrogliosis centered on the corticomedullary junction. Nine months after the operation, he only had one seizure due to a decrease in the dose of PER (Engel class ID).
Patient 2
A 62-year-old man underwent resection of an anaplastic astrocytoma in the left frontal lobe through a left fronto- parietal craniotomy at 34 years of age. He underwent radiation therapy (whole brain 40 Gy, local 20 Gy) and chemotherapy, including interferon-β and nimustine hydrochloride. No subsequent tumor recurrence was observed. He never experienced seizures and did not take any AED.
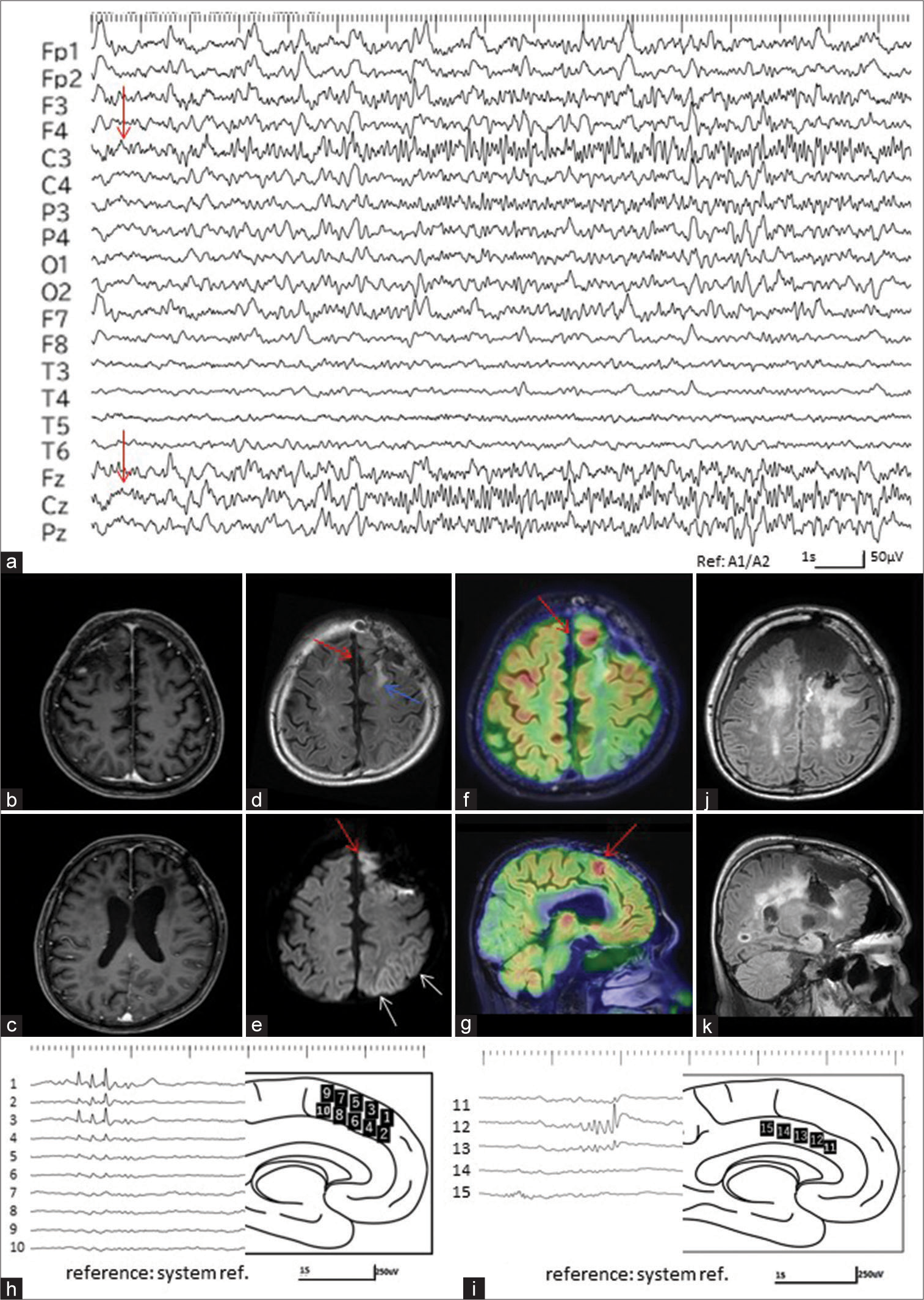
However, he had a generalized seizure for the 1st time at the age of 60 years. Intravenous administration of DZP and LEV stopped his seizures, but impaired consciousness was subsequently prolonged. EEG revealed ictal discharges with evolution continued on the medial part of the left frontal region [
Figure 2:
(Patient 2) (a) Electroencephalogram (EEG) at the 1st super-refractory status epilepticus depicts ictal discharges with evolution continued on the medial part of the left frontal region at the C3 and Cz (red arrows). (b and c) T1-weighted images with gadolinium enhancement fail to reveal the tumor recurrence in the left frontal lobe. (d) Fluid attenuated inversion recovery (FLAIR) image shows gliosis at the interhemispheric cortex medial to the resection cavity (red arrow), in addition to the frontal white matter posterior to the resection cavity (blue arrow). (e) Diffusion-weighted images depict a strong hyperintensity at the medial part of the resection cavity in the left frontal lobe (red arrow) and laminar hyperintensity along with the cortex in the left parietal lobe (white arrows). (f and g) Axial (f) and sagittal views (g) of the fusion images of positron emission tomography with fluorodeoxy glucose (FDG-PET) and FLAIR images demonstrate a strong accumulation at the interhemispheric cortex, medial to the resection cavity in the left frontal lobe (red arrows). (h and i) Intraoperative electrocorticography shows frequent paroxysmal discharges on the interhemispherical surface, medial to the surgical defect in the left frontal lobe. The location and number of the electrodes are indicated on the schematic drawing of the interhemispheric surface. (j and k) Axial (j) and sagittal views (k) of FLAIR images, immediately after the operation, show the resection area, which is identical to the high accumulation area on FDG-PET.
Nine months after the initial SRSE, the patient had an aphasia attack with impaired consciousness. EEG demonstrated repeated ictal discharges at the medial part of the left frontal region, as observed at the 1st SRSE. Treatment for this NCSE required 7 days of general anesthesia with MDZ, in addition to intravenous fPHT.
He developed the same NCSE in the 4th month after the 2nd SRSE. In addition, EEG showed repeated ictal discharges at the medial side of the left frontal region. Eleven days of general anesthesia with MDZ were required to control the 3rd SRSE.
Based on the EEG findings corresponding to the 3 SRSEs and the concordant DWI and FDG-PET findings at the 1st SRSE, we considered that there was an epileptogenic region at the medial part of the left frontal lobe. Three months after recovery from the 3rd SRSE, during the interictal period, reopening of the fronto-parietal craniotomy was performed under TIVA with propofol. Intraoperative ECoG showed frequent paroxysmal discharges on the interhemispheric surface, medial to the surgical defect in the left frontal lobe [
Postoperative mild right hemiparesis and aphasia were transiently observed, but no seizures occurred. On the 20th postoperative day, he was transferred to a rehabilitation hospital with prescriptions of LEV, LCM, VPA, and CLB. Pathological examination of the resected frontal lobe showed marked astrogliosis, but no tumor recurrence was observed. Postoperative EEG failed to reveal a paroxysmal discharge. No seizures occurred during the 3-year postoperative follow- up (Engel class IA).
DISCUSSION
In both patients with SRSE, good seizure outcomes were obtained by performing focal resection surgery. In Patient 1, resection surgery was performed during the super- refractory seizure episode to recover from SRSE. In Patient 2, the purpose of the surgery was to prevent recurrence of SRSE, which occurred 3 times in 1 year and 1 month, and the surgery was performed during the interictal period at 3 months after recovery from the 3rd SRSE.
In both patients, the EEG findings during SRSE, that is, the “ictal” EEG findings, were the most useful for the preoperative identification of the epileptogenic region, confirming the previous reports.[
In resection surgery during SRSE, such as in Patient 1, it was seen that it is highly possible to identify the epileptogenic region by intraoperative ECoG, instead of gold standard chronic intracranial EEG recording, and most of the reported cases achieved good seizure outcomes with the guidance of intraoperative ECoG recording.[
In Patient 2, since the surgery was performed during the interictal state, chronic intracranial EEG recording can be indicated as a presurgical evaluation. However, we selected intraoperative ECoG recording for guidance because it was anticipated that the 4th SRSE would be induced with the reduction of AED and electrical stimulation during functional mapping. In addition, the fact that the ictal EEG findings, which were consistent during all three SRSEs, were concordant with DWI and FDG-PET findings at the first SRSE was also one of the reasons for the selection of intraoperative ECoG guidance.
Regarding the identification of the non-eloquent cortex, in Patient 1, the preoperative evaluation of the initial surgery for MTLE revealed that the dominancy of both language and memory was located on the contralateral side. In Patient 2, although the localization of the dominant hemisphere was not apparent, the site indicated by FDG-PET was the site corresponding to the left supplementary motor area (SMA) or pre-SMA, and it was judged that this site was not eloquent. In fact, in Patient 1, no obvious exacerbation of higher brain function was observed after surgery. Postoperative right hemiparesis and aphasia in Patient 2 were transient, which are characteristic symptoms after resection of the SMA.
Regarding the timing of surgery for SRSE, in the literature, surgical interventions have been performed at least 2 weeks after persistent SE in all but one patient who was operated within 8 days of the onset.[
Both patients suddenly developed SRSE after a long period of time from the initial craniotomy. Patient 1 with MTLE had SRSE after a seizure-free period of 19 years, whereas in Patient 2, with no prior history of seizure, SRSE began to occur 26 years after the first craniotomy. In both cases, strong astrogliosis was histopathologically found in the resected epileptogenic area. After the initial surgery, prolonged astrogliosis progression was observed around the excision area, and the area was considered to have acquired strong epileptogenicity. In Patient 1, hypoplasia of the entire left hemisphere was already observed in the preoperative image of MTLE, and there was originally a pathological condition that caused epilepsy not only in the medial temporal lobe but also in the lateral temporal lobe and frontal lobe.
CONCLUSION
Although the number of cases presented here is minimal (two patients), the present findings provide additional support for the recent idea that focal resection can be indicated for SRSE, in which a well-localized epileptogenic area is identified in the non-eloquent cortex with EEG and intraoperative ECoG.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflict of interest.
References
1. Abend NS, Dlugos DJ. Treatment of refractory status epilepticus: Literature review and a proposed protocol. Pediatr Neurol. 2008. 38: 377-90
2. Dubey D, Kalita J, Misra UK. Status epilepticus: Refractory and super-refractory. Neurol India. 2017. 65: S12-7
3. Ferlisi M, Shorvon S. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain. 2012. 135: 2314-28
4. Gorman DG, Shields WD, Shewmon DA, Chugani HT, Finkel R, Comair YG. Neurosurgical treatment of refractory status epilepticus. Epilepsia. 1992. 33: 546-9
5. Hocker S, Nagarajan E, Rabinstein AA, Hanson D, Britton JW. Progressive brain atrophy in super-refractory status epilepticus. JAMA Neurol. 2016. 73: 1201-7
6. Kirmani BF, Au K, Ayari L, John M, Shetty P, Delorenzo RJ. Super-refractory status epilepticus: Prognosis and recent advances in management. Aging Dis. 2021. 12: 1097-119
7. Lhatoo SD, Alexopoulos AV. The surgical treatment of status epilepticus. Epilepsia. 2007. 48: 61-5
8. Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF. Refractory status epilepticus: Frequency, risk factors, and impact on outcome. Arch Neurol. 2002. 59: 205-10
9. Ochoa JG, Dougherty M, Papanastassiou A, Gidal B, Mohamed I, Vossler DG. Treatment of super-refractory status epileptics: A review. Epilepsy Curr. 2021. 21: 405-15
10. Rai S, Drislane FW. Treatment of refractory and super-refractory status epilepticus. Neurotherapeutics. 2018. 15: 697-712
11. Sakata A, Mukae N, Morioka T, Tanaka S, Shimogawa T, Shigeto H. Simultaneous electroencephalographic and electocorticographic recordings of lateralized periodic discharges in temporal lobe epilepsy. Clin EEG Neurosci. 2022. 53: 61-9
12. Samanta D, Garrity L, Arya R. Refractory and super-refractory status epilepticus. Indian Pediatr. 2020. 57: 239-53
13. Shimogawa T, Morioka T, Sayama T, Haga S, Kanazawa Y, Murao K. The initial use of arterial spin labeling perfusion and diffusion-weighted magnetic resonance images in the diagnosis of nonconvulsive partial status epilepticus. Epilepsy Res. 2017. 129: 162-73
14. Shirozu N, Morioka T, Tokunaga S, Shimogawa T, Inoue D, Arihiro S. Comparison of pseudocontinuous arterial spin labeling perfusion MR images and time-of-flight MR angiography in the detection of periictal hyperperfusion. eNeurological Sci. 2020. 19: 100233
15. Shorvon S, Ferlisi M. The treatment of super-refractory status epilepticus: A critical review of available therapies and a clinical treatment protocol. Brain. 2011. 134: 2802-18
16. Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S. A definition and classification of status epilepticus report of the ILAE task force on classification of status epilepticus. Epilepsia. 2015. 56: 1515-23
17. Uysal U, Landazuri P, Pearson C, Mittal M, Hammond N. Unexpected aphasia following right temporal lobectomy as treatment of recurrent super-refractory status epilepticus. Case Rep Neurol. 2017. 9: 195-203