- Department of Neurosurgery, University of Erlangen-Nuremberg, Erlangen-Nuremberg, Germany
- Department of Neurosurgery, Hannover Nordstadt Hospital, Hannover, Germany
- Department of Neurosurgery, Bundeswehrkrankenhaus Ulm, Ulm, Germany
Correspondence Address:
Levent Tanrikulu
Department of Neurosurgery, University of Erlangen-Nuremberg, Erlangen-Nuremberg, Germany
Department of Neurosurgery, Bundeswehrkrankenhaus Ulm, Ulm, Germany
DOI:10.4103/2152-7806.195572
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Levent Tanrikulu, Peer Lohse, Rudolf Fahlbusch, Ramin Naraghi. Hearing preservation in acoustic neuroma resection: Analysis of petrous bone measurement and intraoperative application. 12-Dec-2016;7:
How to cite this URL: Levent Tanrikulu, Peer Lohse, Rudolf Fahlbusch, Ramin Naraghi. Hearing preservation in acoustic neuroma resection: Analysis of petrous bone measurement and intraoperative application. 12-Dec-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/hearing-preservation-in-acoustic-neuroma-resection-analysis-of-petrous-bone-measurement-and-intraoperative-application/
Abstract
Background:There is an increased risk for labyrinthine injury for the resection of acoustic neuromas (AN) on the suboccipital, retrosigmoid approach. Prognostic factors should be analyzed for the postoperative hearing function.
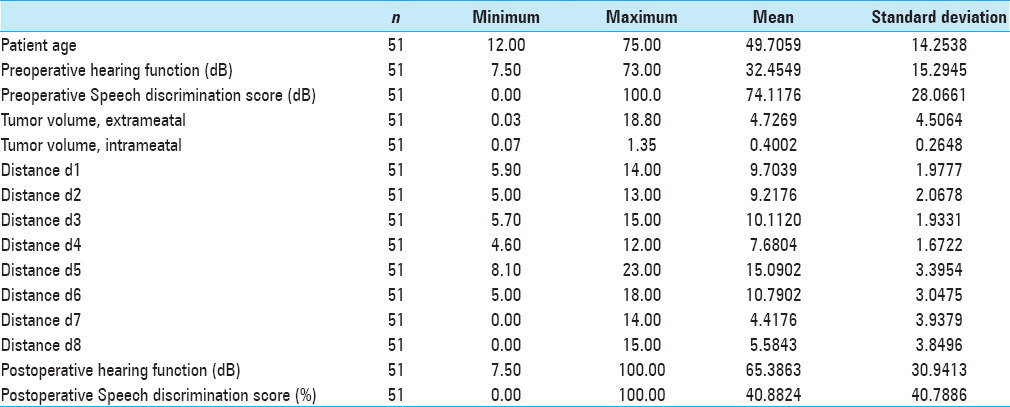
Methods:We examined 51 patients with ANs using preoperative intact hearing function. Audiological data were obtained by pure tone audiogram (PTA) and speech audiogram. The preoperative and postoperative anatomical localization of the labyrinth was measured with specific distances regarding the tumor and corresponding anatomy of the posterior fossa by high-resolution magnetic resonance imaging (MRI).
Results:Postoperative MRI controls confirmed no injuries to the labyrinth (0%). The postoperative hearing results showed 100% hearing preservation for T1-tumors (8 ml/>2.3 cm). Postoperative deafness was seen in all cases with ventral tumor extension higher than 5.5 mm. Postoperative loss of hearing was seen in all cases with hearing preservation with 6–8% of speech discrimination and an increase in the hearing threshold of 12 dB in the PTA compared to the preoperative hearing status.
Conclusion:Petrous bone measurement by high-resolution MRI data enables safe surgical exposure of the internal acoustic canal with avoidance of injury to the labyrinth and a better postoperative prognosis, especially for intrameatal ANs and for the resection of intrameatal portions of larger neuromas. The prognostic factors enable the patients and the surgeon a better estimation of postoperative results regarding deafness and postoperative hypacusis and support a consolidated treatment planning.
Keywords: Acoustic neuroma, hearing preservation, high resolution MRI, petrous bone measurement
INTRODUCTION
Exposure of the internal acoustic canal (IAC) has significant risks for injury to the labyrinth for the resection of acoustic neuromas (AN) on the suboccipital, retrosigmoid approach. Former studies showed higher incidences of injury for very medially localized semicircular canals of the labyrinth.[
MATERIALS AND METHODS
Clinical data
51 patients (male/female: 42/58%, minimum age: 12, maximum age: 75, mean age: 49) with AN with confirmed and intact preoperative hearing function underwent tumor resection after suboccipital, retrosigmoid craniectomy. One patient (2%) had Type 2 Recklinghausen's disease. Symptoms such as vertigo, tinnitus, ataxia, preoperative hypacusis, headache, and cranial nerve dysfunctions were documented preoperatively. Inclusion criteria were confirmed hearing function on the side of the tumor (see below audiologic parameters), preoperative and postoperative determination of hearing threshold levels (in dB) and speech discrimination, preoperative high-resolution MRI with measurements of the petrous bone, and postoperative MRI after 3–6 months to look for injury of the labyrinth. Exclusion criteria were recurrent ANs, radiated tumors, and other histological entities such as meningiomas in the cerebellopontine angle (CPA).
Audiologic parameters
Four hearing classes A–D were defined according to the Committee on Hearing and Equilibrium of the AAO-HNSF: A – hearing threshold in audiogram <30 dB and language discrimination score >70% (within 70–100 dB), B – hearing threshold >30 dB and <50 dB and language discrimination >50%, C – hearing threshold >50 dB and language discrimination >50%, D – all hearing threshold values, and language discrimination <50%.[
Preoperative hearing function
A verifiable hearing function was a prerequisite for inclusion in our study. Forty-seven percent of our patients were assigned to hearing class A, 33% to class B, 10% to class C, and 10% to class D.
Imaging
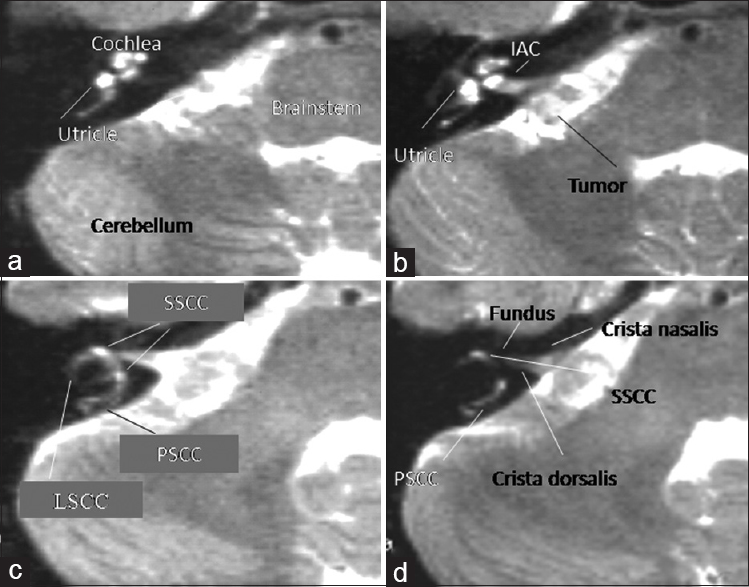
All patients underwent high-resolution 1.5 Tesla MR imaging at the Department of Neuroradiology at the University of Erlangen-Nuremberg (Siemens Magnetom, T1w: TR 0.6, TE 20, slice thickness 3 mm, matrix 256 × 256; T2w: TR 2.500, TE 45–90, slice thickness 3 mm, matrix 256 × 256). In high-resolution T2 weighted images, all three semicircular canals of the labyrinth, the utricule, and the cochlea are delineated within the petrous bone in correspondence to the IAC. Especially the posterior semicircular canal (PSCC) can be localized quite medially and directly under the surface of the petrous bone, and therefore, can be prone to injury by exposure of the IAC, as shown in
Figure 2
(a-d) Ultra high-resolution T2-weighted MR slice images of a patient with right-sided AN from caudal to cranial direction a>d. Note that the PSCC in (d) is localized quite medially and near to the IAC, so that it might be prone to injury after exposure of the IAC (T2w: T2-weighted magnetic resonance imaging, AN: Acoustic neuroma, IAC: Internal acoustic canal, PSCC: Posterior semicircular canal, LSCC: Lateral semicircular canal, SSCC: Superior semicircular canal)
Principles of petrous bone measurement
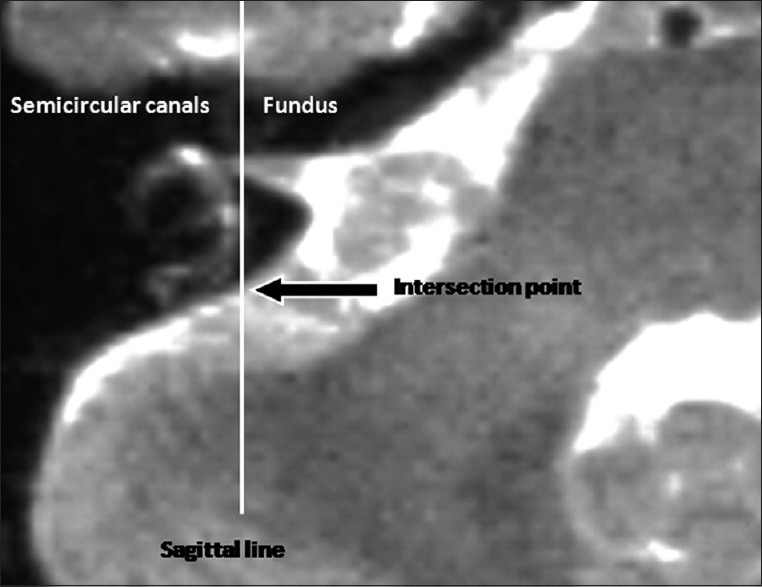
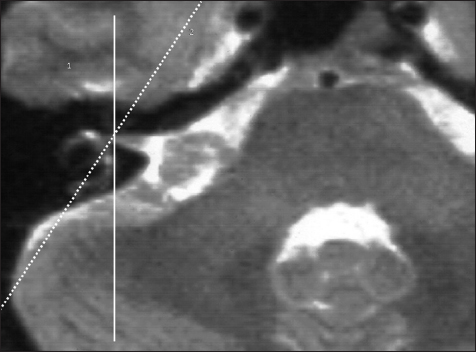
The surgeon looks from the dorsolateral aspect of the posterior fossa onto the petrous bone and can see the tumor spreading out of the IAC. For opening of the IAC, a portion of the petrous bone has to be removed without having any safe and clear macroscopic landmarks for the localization of the underlying labyrinth. Therefore, we choose the transversal, highly T2-weighted image slices, which delineate the medially localized portion of the semicircular canals in high resolution, mostly the PSCC. A sagittal line is constructed into this slice, which courses through the fundus of the IAC and tangentially touches the most medial aspect of the semicircular canals [
Figure 3
High resolution T2-weighted image slice for the measurement of the anatomical localization of the labyrinth in projection to the dorsal surface of the petrous bone. A sagittal line is constructed through the fundus of the IAC, which tangentially touches the most medially localized portion of the semicircular canals of the labyrinth. The distance of the resulting intersection point from the posterior margin of the IAC can be measured intraoperatively with a ruler
Figure 4
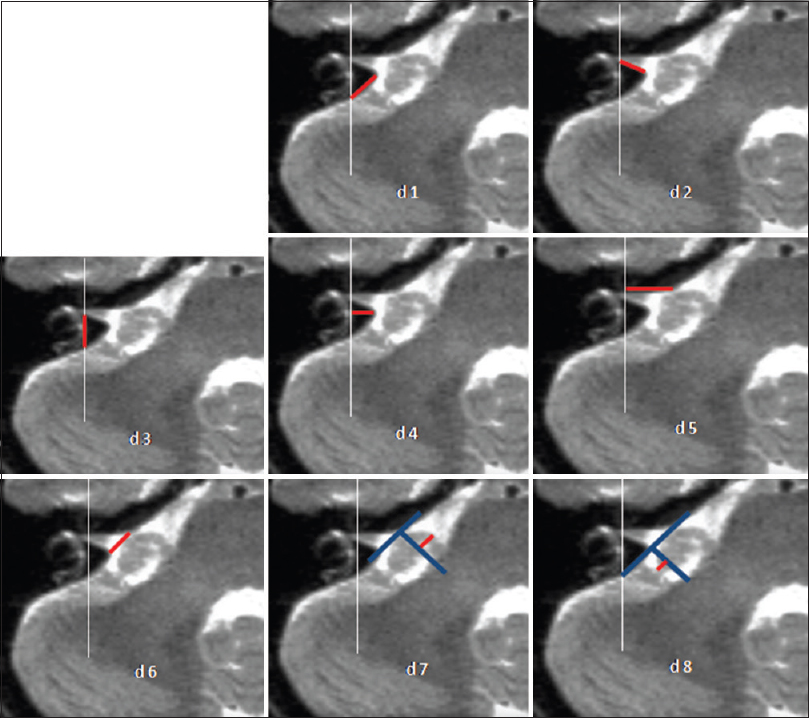
Anatomical measurement of distances d1 to d8 with high resolution T2 weighted images. d1: Distance from Crista dorsalis (dorsal margin) of the IAC to the intersection point of the medial limitation of the labyrinth with the dorsal surface (facies dorsalis) of the petrous bone. d2: Crista dorsalis of the IAC to the fundus. d3: Fundus to the intersection point of the medial limitation of the labyrinth with the facies dorsalis of the petrous bone. d4: Perpendicular line from the sagittal line of the medial limitation of the labyrinth to the crista dorsalis of the IAC. d5: Distance from the Crista nasalis of the IAC to the fundus. d6: Distance from the Crista nasalis to the Crista dorsalis of the IAC. d7: Nasal extension of the tumor over the Crista nasalis of the IAC. d8: Dorsal extension of the tumor over the Crista dorsalis of the IAC
Calculation of the tumor volume
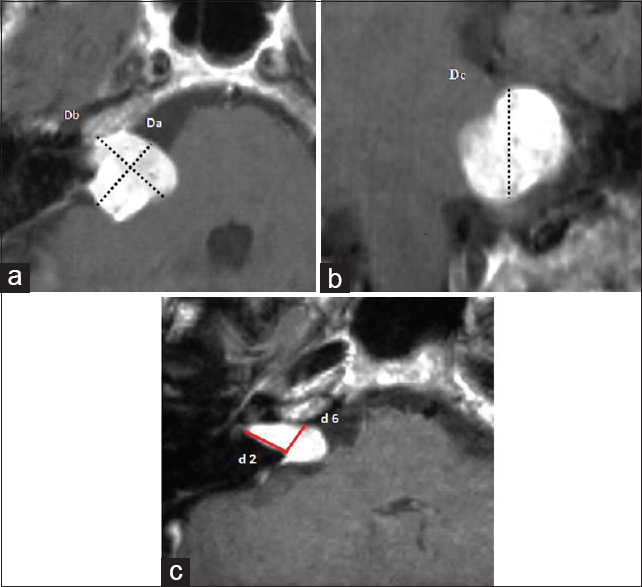
Extrameatal tumor volume: The extrameatal tumor volume is defined by the volume of a sphere: V =4/3 × π × r3. Three diameters, namely, Da, Db, and Dc, from the T1-weighted images in transversal, coronal, and sagittal planes are measured. Note the three diameters Da (parallel to the petrous bone), Db (perpendicular to Db), and Dc (maximal vertical diameter of the tumor from the coronal plane; see Figures V = 4/3 × π × (Da/2 × Db/2 × Dc/2). Intrameatal tumor volume: The intrameatal tumor volume is defined by the volume of a conus: V =1/3 × r2 × π × h. r is represented by the half of the previously described distance d6 (see petrous bone measurements, see above), and h is the height of the conus represented by the previously describes distance d2 [
Figure 5
(a, b) Measurement of the extrameatal tumor volume. (a) Transversal image slice with diameters Da (parallel to the petrous bone) and Db perpendicular to Da. (b) Maximal vertical tumor diameter Dc from the coronal plane. (c) Measurement of the intrameatal tumor volume. d2: Crista dorsalis of the IAC to the fundus. d6: Distance from the Crista nasalis to the Crista dorsalis of the IAC
The total tumor volume results from the sum of the extrameatal and intrameatal tumor volumes:
V = 4/3 × π × (Da/2 × Db/2 × Dc/2) +1/3× (d6/2)2 × π × d2
Definition of tumor classes
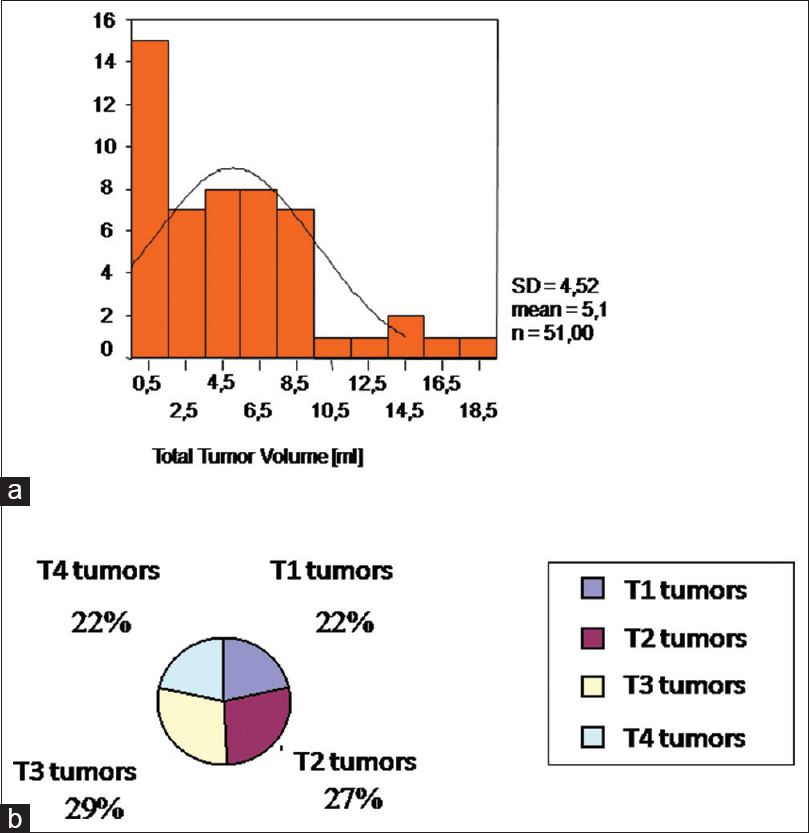
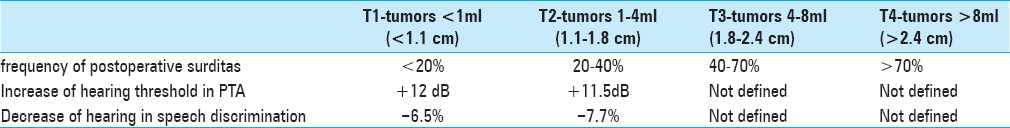
The tumors were defined by four size categories, T1–T4, in order to evaluate possible or not possible prognosis regarding the expecting postoperative hearing preservation or deafness for each tumor class:
T1: < 1 ml (<1.1 cm diameter)
T2: 1 ml–4 ml (1.1–1.8 cm diameter)
T3: 4 ml–8 ml (1.8–2.4 cm diameter)
T4: >8 ml (>2.4 cm diameter).
Figure 7
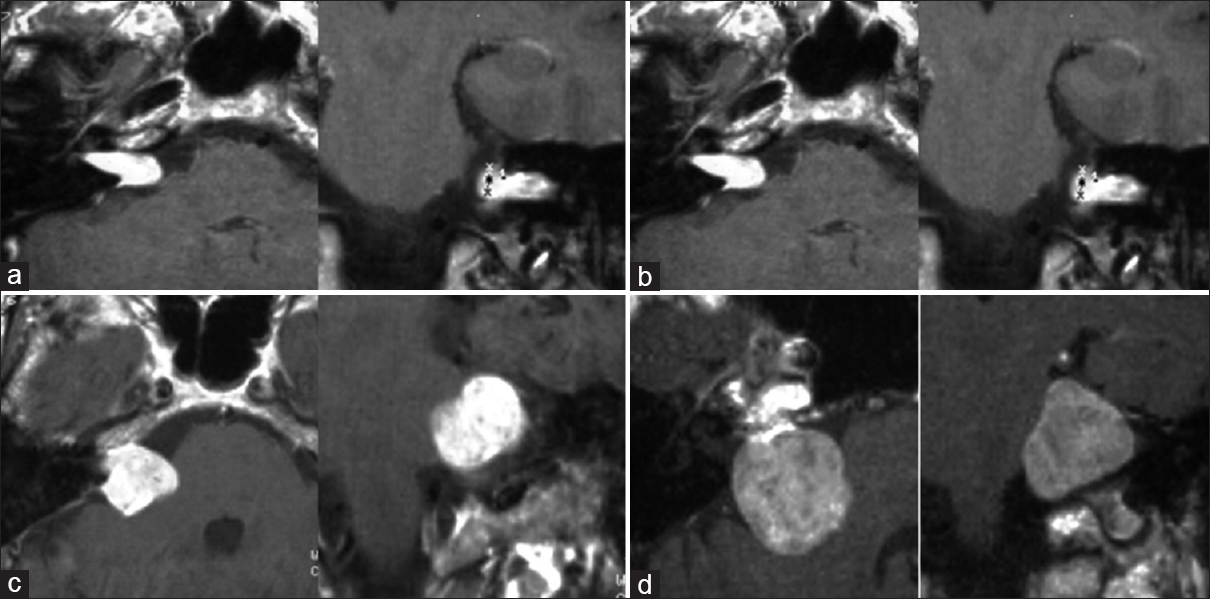
Examples of examined and resected tumors according to the defined tumor classes T1-T4. (a) small T1 tumor without contact to the brainstem, 0.42 ml. (b) T2 tumor with contact to the brainstem without significant compression, 3.6 ml. (c) T3 tumor with brainstem compression, 5.6 ml. (d) T4 tumor with compression and dislocation of the brainstem, 13 ml
Postoperative imaging for evaluation of labyrinth preservation and tumor recurrence
All patients underwent postoperative MRI 3–6 months after the surgery [
Surgical technique
The suboccipital, retrosigmoid approach was performed in all 51 patients. The patients were positioned with elevation of the ipsilateral shoulder and the head turned horizontally to the contralateral side. Cerebrospinal fluid (CSF) was released after craniectomy from the cisterna magna. After this procedure, one can see the tumor growing out of the IAC. As a next step, intracapsular shrinking of extrameatal tumor portion starts. After the extrameatal tumor shrinkage, the opening of the IAC is delineated with the Crista dorsalis. The distance d1, which is gained by the preoperative T2-weighted images, is marked on a sterile ruler and transferred to the surgical field that shows the distance from the posterior border of the IAC (Crista dorsalis) to the medial limitation of the labyrinth on the dorsal surface of the petrous bone for secure exposure of the IAC [
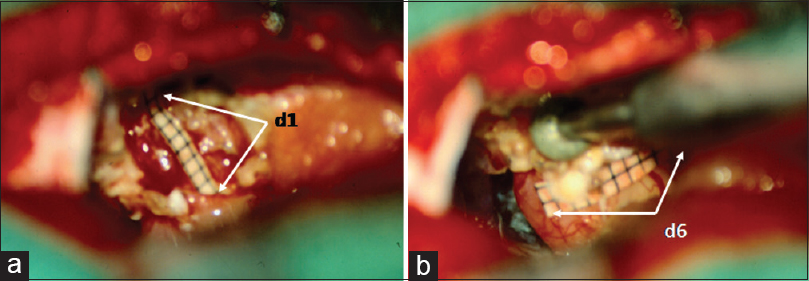
Figure 9
Microsurgical view onto the CPA with depiction of the distances d1 and d6. (a) d1: Distance from Crista dorsalis – dorsal margin – of the IAC to the intersection point of the medial limitation of the labyrinth with the dorsal surface of the petrous bone. (b) d6: Distance from the Crista nasalis to the Crista dorsalis of the IAC
RESULTS
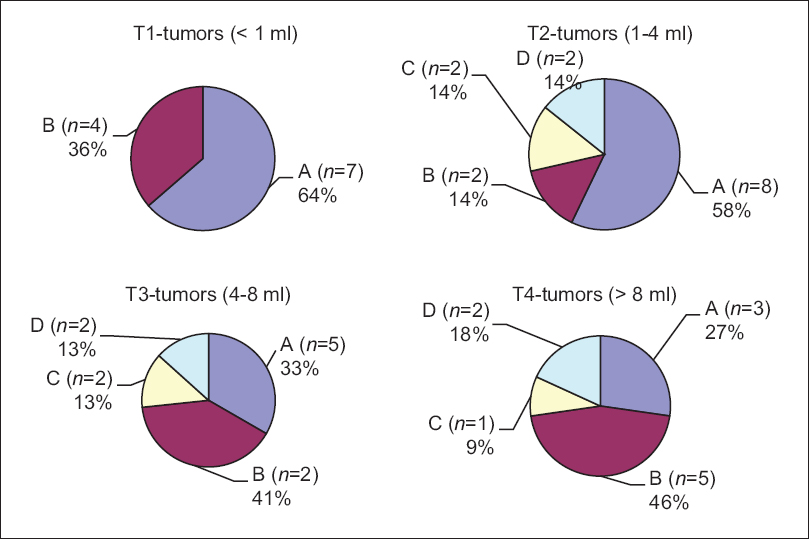
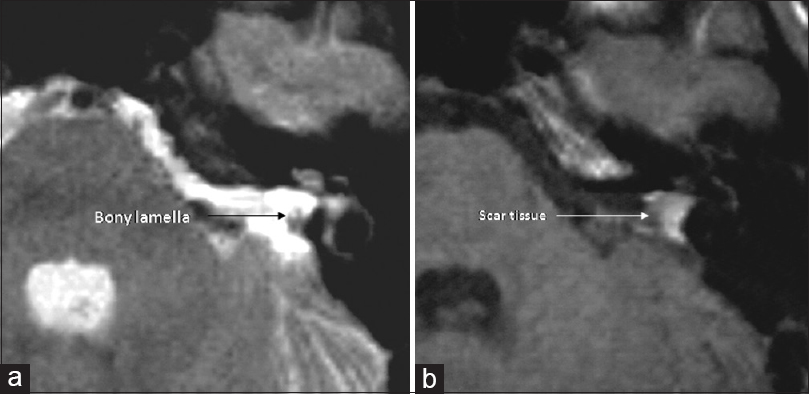
There was no mortality intra or postoperatively (0.0%). Two out of 51 patients (3.9%) developed transient CSF leak, which was observed conservatively and diminished spontaneously. One patient had immediate postoperative intracerebral hemorrhage (1.96%) within the CPA and underwent emergent revision with good neurological recovery. In 20 of 51 patients (39%), we saw postoperative facial nerve paresis; in 25% of the patients with mild pareses House–Brackmann II°-III° with good remission in follow-up and in 14% of the patients (n = 7) with House-Brackmann grades IV°-V°. The preservation of the labyrinthine system could be achieved with the method of petrous bone measurement in all 51 patients (100%). A bony lamella of 1–2 mm thickness stayed to the medial border of the semicircular canals in all 51 cases (100%). Tumor recurrences were not seen on the postoperative MRI scans 3–6 months after the surgery (0.0%). Two hearing tests (pure tone audiogram, PTA and speech discrimination score, SDS) were performed within the first two postoperative weeks. In 26 of 51 patients (51%), a useable hearing function was available with 18% with normal hearing function (Class A), in 27% with impaired but useable hearing function (Class B), and 6% of the patients had a significantly limited function but useful for the localization of noises and language comprehension (Class C). In all patients of Class C, the application of a hearing aid supported the hearing function because of intact speech discrimination. Postoperative deafness was seen in 25 of 51 patients (49%; Class D). For T1-tumors (<1 ml/<1.1 cm), we saw very good and moderate good hearing function with 36% of T1-tumor patients with Class A and 64% with Class B quality.
In T2-tumors (1–4 ml/1.1–1.8 cm), the success rate was reduced to 50% of not useable hearing function (Class D), 7% to Class C, 29% in Class B, and 14% in Class A. In larger tumors (>8 ml), the success rate was significantly reduced <20% of effective hearing preservation.
For T3-tumors (4–8 ml/1.8–2.3 cm), in 40% of the patients, postoperative hearing function was preserved with 20% Class A quality, 13% Class B, and 7% Class C. For T4-tumors (4–8 ml/>2.3 cm) deafness was seen in 80% of cases (Class D) and 9% moderate to useable hearing function in each Classes A–C. The results of the measured clinical, audiological, and anatomical parameters are shown by
DISCUSSION
Functional hypacusis after resection of acoustic neuromas, even in anatomically well preserved cochlear nerve is a disappointing event for each surgeon by preparation of the tumor at the cochlear nerve with coagulation of small vessels and subsequent ischemic events and nerval damage by vibration and hyperthermia of the petrous bone during drilling of the posterior elements of the petrous bone and affection of the labyrinthine system. Multiple possibilities were introduced into this field during surgery.[
We saw a significant relationship between preoperative hearing function and postoperative hearing result for all tumor sizes in our series (P < 0.05) for the pre and postoperative PTA and the speech audiogram for the T1-tumors (<1 ml/<1 cm). If the tumor is localized primarily intrametally, the postoperative hearing preservation is nearly equal to the preoperative hearing level with a frequency of more than 80% (80–83%). If the tumor is small and primarily extending extrameatally, the postoperative hearing quality is reduced for one hearing class (in our series Class B). For patients with larger tumors (T3 and T4: >4 ml/>2.3 cm), there is no relationship between preoperative hearing function and postoperative hearing result. Obviously, there are other factors, e.g., intraoperative lesions during extended preparation or preoperative tumor-associated lesion of the cochlear nerve in the audiogram for the T3 and T4-tumors. The difference is primarily noticed in a worsened speech discrimination. A possible cause for this significant difference might be the different influence of the tumor on the blood supply of the cochlear nerve intra and extrameatally. For T2-tumors (1–4 ml/1.1–1.8 cm), we saw a narrow correlation of total tumor volume and especially speech discrimination (P < 0.01). The risk for postoperative deafness for T2-tumors was 20–45%, whereas the intrameatal tumor portion did not play a role. In T3 (4–8 ml/1.8–2.3 cm) and T4-tumors (>8 ml/>2.3 cm), there was no correlation with the preoperative data, whereas the risk for postoperative deafness was 50–80% for T3-tumors and >80% for T4-tumors. In the analysis of the petrous bone and tumor measurements, we saw a very interesting and highly significant influencing factor (P < 0.001), the ventral tumor extension above the Crista nasalis of the IAC. From our data, we can give a superior limit of 5.5 mm for the ventral tumor extension above the Crista nasalis in our patient population where postoperative deafness occurred independent of the tumor size.
In this study, we saw a very good postoperative prognosis for the small tumors (<2 ml/<1.4 cm) as a good argument for an early, surgical resection, whereas stereotactic radiosurgery was established with excellent hearing preservation rates of 60–75% and reduced morbidity, especially for patients with preoperative good hearing function and medium-sized and large tumors as an appropriate alternative.[
We could implement the method of preoperative petrous bone measurement into the surgical setup. By the transfer of preoperatively acquired individual, anatomical data from high-resolution MRI to the operative field we could avoid any injury to the labyrinthine system on the suboccipital, retrosigmoid approach in a prospective manner. We think that this gives the possibility to reduce an important risk factor of postoperative hearing preservation, especially in small, intrameatal tumors. Of course, gammaknife radiosurgery is an excellent alternative with excellent risk profile and outcome statistics in comparison to our work. With our study we were able to give a prognosis of postoperative hearing preservation, which contributed to patient and physician relationship as well as patient consultation.
CONCLUSION
Petrous bone measurement by high-resolution MRI data enables the safe surgical exposure of the IAC with avoidance of injury to the labyrinthine system along with a better postoperative prognosis especially for intrameatal acoustic neuromas and the resection of intrameatal portions of larger neuromas. The prognostic factors enable the patients and the surgeon a better estimation of postoperative results regarding deafness and postoperative hypacusis and support a consolidated treatment planning.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. . American Academy of Otolaryngology. Head and Neck Surgery Foundation INC: Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). Otolaryngol Head Neck Surg. 1995. 113: 179-80
2. Brackmann DE, House JR, Hitselberger WE. Technical modifications to the middle fossa craniotomy approach in removal of acoustic neuromas. Am J Otol. 1994. 15: 614-9
3. Cohen NL, Ransohoff J. Hearing preservation: Posterior fossa approach. Otolaryngol Head Neck Surg. 1984. 92: 176-83
4. Comey CH, Jannetta PJ, Sheptak PE, Joh HD, Burkhart LE. Staged removal of acoustic tumors: Techniques and lessons learned from a series of 83 patients. Neurosurgery. 1995. 37: 915-20
5. Discroll CL, Jackler RK, Pitts LH, Banthia V. Is the entire fundus of the internal auditory canal visible during the middle fossa approach for acoustic neuroma?. Am J Otol. 2000. 21: 382-8
6. Fischer G, Fischer C, Remond J. Hearing preservation in acoustic neurinoma surgery. J Neurosurgery. 1992. 76: 910-7
7. Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988. 97: 55-66
8. Koos WT, Day JD, Matula C, Levy DI. Neurotopographic considerations in the microsurgical treatment of small. J Neurosurgery. 1998. 88: 506-12
9. Kumon Y, Sakaki S, Kohno K, Ohta S, Nakagawa K, Ohue S. Selection of surgical approaches for small acoustic neurinomas. Surg Neurol. 2000. 53: 52-9
10. Low WK. Enhancing hearing preservation in endoscopic-assisted excision of acoustic neuroma via the retrosigmoid approach. J Laryngol Otol. 1999. 113: 973-7
11. Matthies C, Samii M, Krebs S. Management of vestibular schwannomas (acoustic neuromas): Radiological features in 202 cases--their value for diagnosis and their predictive importance. Neurosurgery. 1997. 40: 469-81
12. Pollock BE, Lunsford LD, Kondziolka D, Flickinger JC, Bissonette DJ, Kelsey SF. Outcome analysis of acoustic neuroma management: A comparison of microsurgery and stereotactic radiosurgery. Neurosurgery. 1995. 36: 215-24
13. Post KD, Eisenberg MB, Catalano PJ. Hearing preservation in vestibular schwannoma surgery: What factors influence outcome?. J Neurosurg. 1995. 83: 191-6
14. Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): Surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery. 1997. 40: 1-23
15. Sampath P, Rini D, Long DM. Microanatomical variations in the cerebellopontine angle associated with vestibular schwannomas (acoustic neuromas): A retrospective study of 1006 consecutive cases. J Neurosurg. 2000. 92: 70-8
16. Sridhar K, Ramamurthi R, Vasudevan MC, Ramamurti B. Magnetic resonance imaging artifact following acoustic neurofibroma surgery--Case report. Neurol Med Chir. 1999. 39: 938-40
17. Staecker H, Nadol JB Jr, Ojeman R, Ronner S, McKenna MJ. Hearing preservation in acoustic neuroma surgery: Middle fossa versus retrosigmoidal approach. Am J Otol. 2000. 21: 399-404
18. Tatagiba M, Samii M, Matthies C, al Azm M, Sch önmayr R. The significance for postoperative hearing of preserving the labyrinth in acoustic neurinoma surgery. J Neurosurg. 1992. 77: 677-84
19. Yokoyama T, Uemura K, Ryu H, Hinokuma K, Nishizawa S, Yamamoto S. Surgical approach to the internal auditory meatus in acoustic neuroma surgery: Significance of preoperative high-resolution computed tomography. Neurosurgery. 1996. 39: 965-70