- Department of Neurosurgery, Kobe City Medical Center General Hospital, Kobe, Hyogo, Japan.
DOI:10.25259/SNI_387_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Tomoaki Akiyama, Hirotoshi Imamura, Nobuyuki Fukui, Nobuyuki Sakai. Helicobacter cinaedi-infected chronic subdural hematoma mimicking an expanding hematoma: A case report. 14-Jun-2021;12:288
How to cite this URL: Tomoaki Akiyama, Hirotoshi Imamura, Nobuyuki Fukui, Nobuyuki Sakai. Helicobacter cinaedi-infected chronic subdural hematoma mimicking an expanding hematoma: A case report. 14-Jun-2021;12:288. Available from: https://surgicalneurologyint.com/surgicalint-articles/10879/
Abstract
Background: We present the rare case of a spontaneous intracranial subdural empyema caused by Helicobacter cinaedi in a preexisting chronic subdural hematoma (CSDH).
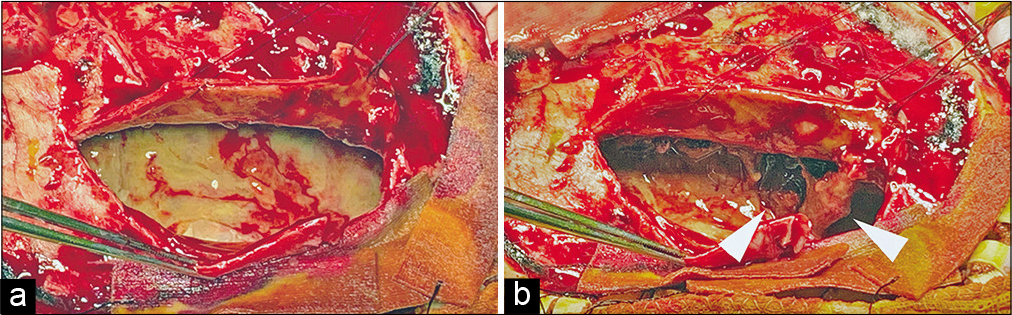
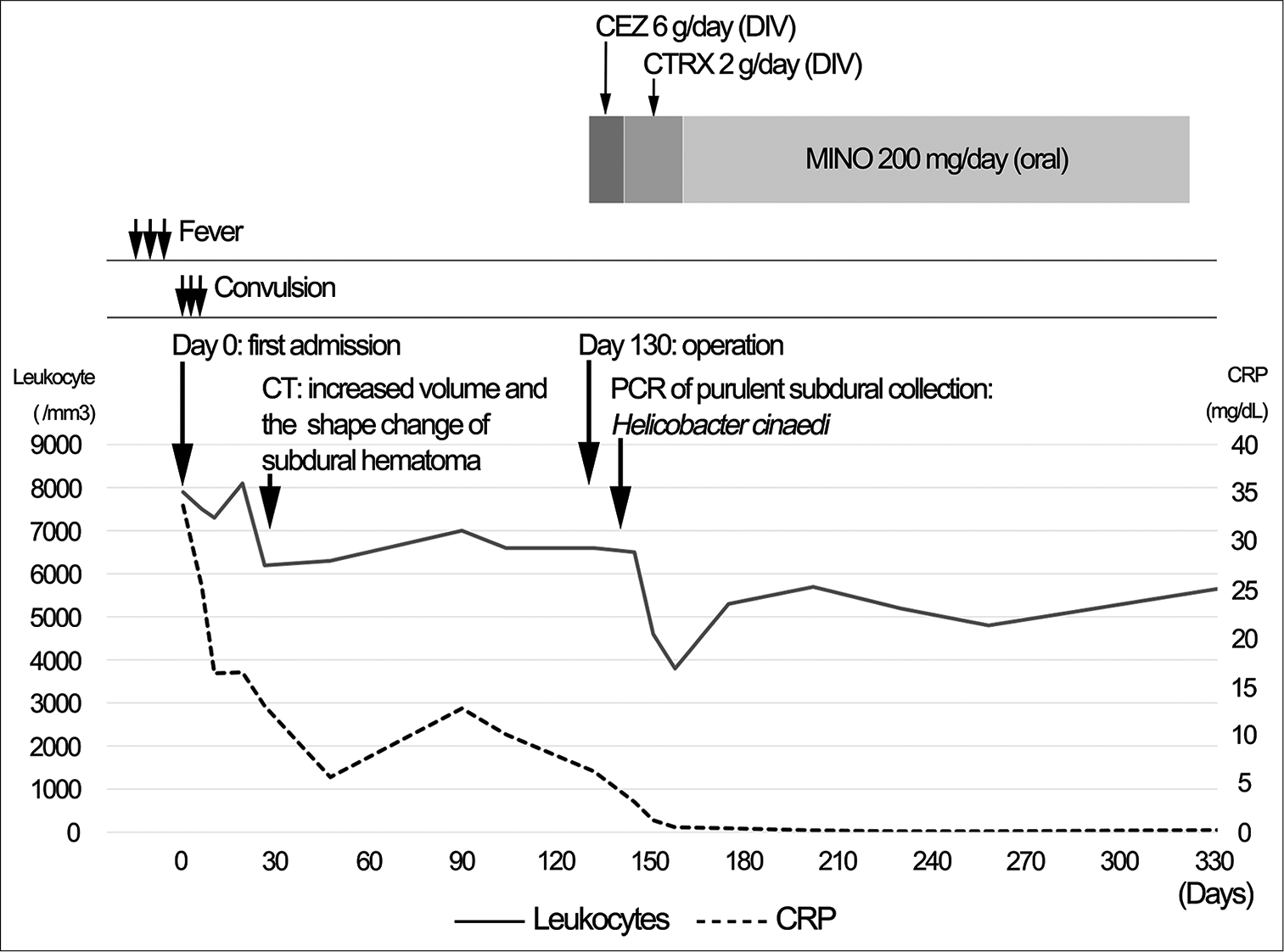
Case Description: A 72-year-old man with a history of the right CSDH that remained radiologically unchanged for the past 2 years with conservative management was transferred to our hospital because of fever and convulsive seizure. Systemic sources of infection were not identified. Fever and extremely high levels of serum C-reactive protein (CRP) spontaneously improved without antibacterial therapy. One month after the fever disappeared, brain computed tomography (CT) showed an increase in CSDH size. Mildly elevated CRP levels persisted without fever. Interval changes in shape on CT and hyperintense signals on diffusion-weighted magnetic resonance imaging (DWI) within the CSDH were observed with no neurological deficits. Five months later, the patient underwent craniotomy for a progressively enlarged CSDH. An infected organized hematoma was found, and copious pus was evacuated. Subsequently, an infected subdural hematoma (ISH) was diagnosed. Although bacterial cultures of the purulent specimen were negative, H. cinaedi was identified by gene sequencing analysis. Six months post antibiotic therapy, the ISH was under control, and abnormal DWI signals disappeared.
Conclusion: To the best of our knowledge, this is the first report of ISH caused by H. cinaedi. This case suggests that ISH can follow a chronic course, mimicking the progressive expansion of subdural hematoma, and that H. cinaedi should be considered as a causative organism of ISH especially when conventional cultures are negative.
Keywords: Chronic subdural hematoma, Helicobacter cinaedi, Infected subdural hematoma, Subdural empyema
INTRODUCTION
Infected subdural hematoma (ISH) caused by hematogenous chronic subdural hematoma (CSDH) infection is a rare clinical entity of intracranial subdural empyema,[
CASE DESCRIPTION
A 72-year-old man with well-controlled type 2 diabetes mellitus, and an implantable cardioverter-defibrillator implantation for Brugada syndrome underwent regular follow-up computed tomography (CT) to evaluate asymptomatic right posttraumatic CSDH. The patient received no anticoagulant drugs. The CSDH remained unchanged over the past 2 years with conservative management.
After 10 days of intermittent fever, the patient was transferred to our hospital because of fever and subsequent seizure in the left upper extremity. On admission, the patient’s temperature was 37.5 °C, and a blood test showed leukocyte levels of 7500/mm3 and C-reactive protein (CRP) levels of 33.26 mg/ dL. Serological tests for human immunodeficiency virus were negative. The patient was not immunocompromised or had not been given immunosuppressive drugs. The brain CT image demonstrated no change in the right CSDH [
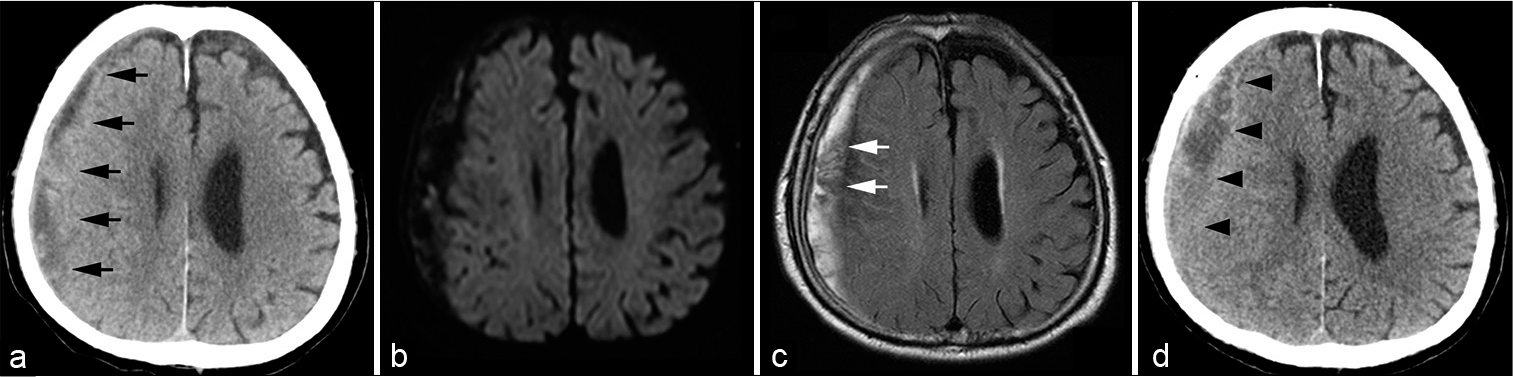
Figure 1:
(a) Head computed tomography (CT) shows right chronic subdural hematoma (CSDH) (black arrows). (b and c) Magnetic resonance imaging demonstrates uniform hyperintensity on diffusion-weighted imaging and hyperintensity with intrahematomal membrane structures on fluid-attenuated inversion recovery (white arrows). (d) One-month follow-up CT after the abatement of fever indicates a slight increase (black arrowheads) in the CSDH size.
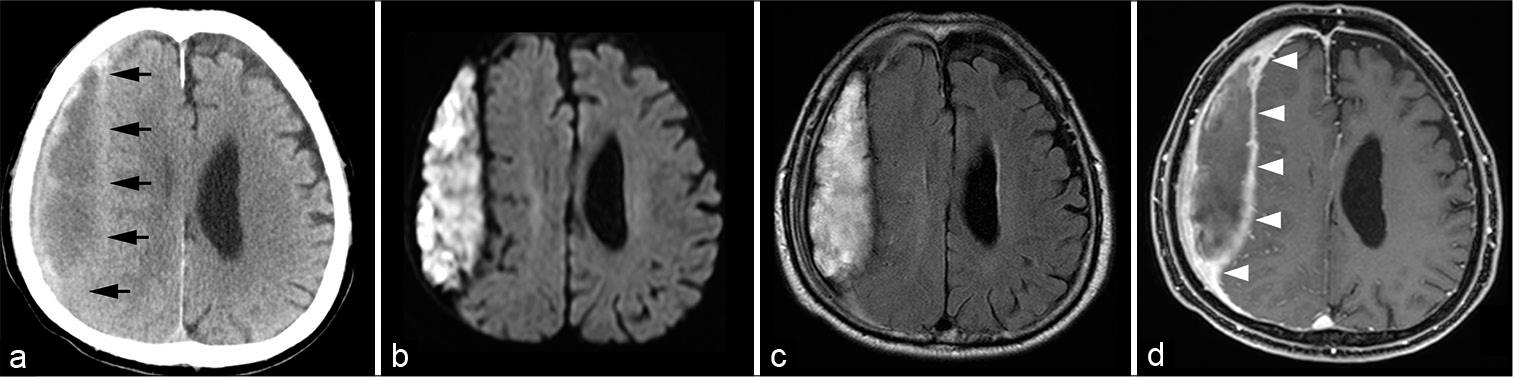
Figure 2:
(a) Head computed tomography reveals a further increase in volume and a biconvex shape of the chronic subdural hematoma (black arrows). (b and c) Magnetic resonance imaging demonstrates an expanding subdural hematoma with no surrounding edema on fluid-attenuated inversion recovery and heterogeneous hyperintense signals corresponding to the expansive component of the subdural hematoma on diffusion-weighted imaging. (d) Postgadolinium T1-weighted image shows enhancement of the thickened hematoma capsule (white arrowheads).
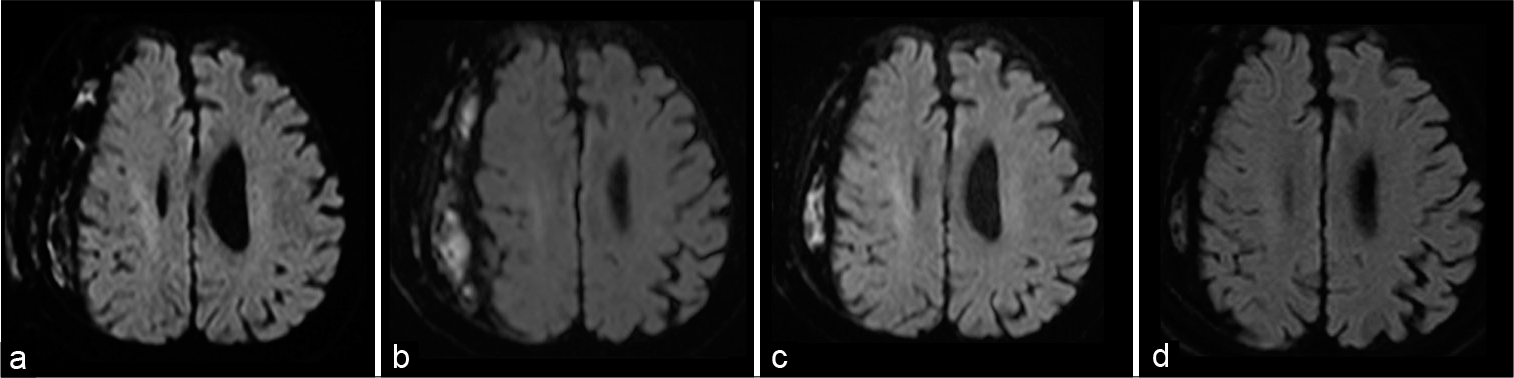
Figure 4:
Head diffusion-weighted magnetic resonance imaging confirms a gradual decrease in the hyperintense signals (indicating the abscess) and, finally, the complete disappearance of the infected subdural hematoma. (a) Two days after surgery, (b) 1 month later, (c) 3 months later, and (d) 6 months later.
DISCUSSION
The present case has two clinically important points. First, subdural empyema developed in the preexisting CSDH and followed a chronic course without neurological deficits. Second, H. cinaedi central nerve system infections should be considered, especially in conventional culture-negative cases.
CSDH is a common disease in neurosurgical practice, while ISH in adults is clinicopathological rare, with only 41 reports available in the literature.[
H. cinaedi is an enterohepatic Gram-negative spiral bacillus first reported by Fennell et al. in 1984.[
Regarding H. cinaedi central nervous system infections, seven reports have been published, including a case of subdural empyema.[
The optimal surgical strategy for ISH has not been clarified because of its rarity.[
CONCLUSION
We describe a rare case of H. cinaedi infection in a preexisting CSDH. ISH can follow a chronic course with mild inflammatory markers, mimicking spontaneous hematoma expansion. In addition, H. cinaedi should be a suspected pathogen, especially when conventional cultures of blood or surgical specimens fail.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abiko S, Nakamura I, Yamaguchi Y, Ohkusu K, Hirayama Y, Matsumoto T. The first case report of cerebral cyst infection due to Helicobacter cinaedi. Jpn J Infect Dis. 2017. 70: 210-12
2. Araoka H, Baba M, Kimura M, Abe M, Inagawa H, Yoneyama A. Clinical characteristics of bacteremia caused by Helicobacter cinaedi and time required for blood cultures to become positive. J Clin Microbiol. 2014. 52: 1519-22
3. Brin C, Sougakoff W, Bielle F, Abi Jaoude S, Bonnet I, Haddad E. Unusual subdural empyema in a homeless patient diagnosed by molecular approach: A case report. BMC Infect Dis. 2020. 20: 357
4. Dabdoub CB, Adorno JO, Urbano J, Silveira EN, Orlandi BM. Review of the management of infected subdural hematoma. World Neurosurg. 2016. 87: 663.e661-8
5. Fennell CL, Totten PA, Quinn TC, Patton DL, Holmes KK, Stamm WE. Characterization of Campylobacterlike organisms isolated from homosexual men. J Infect Dis. 1984. 149: 58-66
6. Hamada J, Ichimura H, Ushio Y. Huge subdural empyema with unusual presentation in infant-case report. Neurol Med Chir (Tokyo). 1993. 33: 40-3
7. Hayashi T, Tomida J, Kawamura Y, Yoshida M, Yokozawa I, Kaneko S. Unusual manifestation of Helicobacter cinaedi infection: A case report of intracranial subdural empyema and bacteremia. BMC Infect Dis. 2017. 17: 40
8. Kawamura Y, Tomida J, Morita Y, Fujii S, Okamoto T, Akaike T. Clinical and bacteriological characteristics of Helicobacter cinaedi infection. J Infect Chemother. 2014. 20: 517-26
9. Konno T, Yamada K, Kasahara S, Umeda Y, Oyake M, Fujita N. Transformation from chronic subdural hematoma into subdural empyema following cat bites: A case report. Rinsho Shinkeigaku. 2015. 55: 657-60
10. Matsumoto T, Goto M, Murakami H, Tanaka T, Nishiyama H, Ono E. Multicenter study to evaluate bloodstream infection by Helicobacter cinaedi in Japan. J Clin Microbiol. 2007. 45: 2853-7
11. Miyake N, Chong Y, Nishida R, Nagasaki Y, Kibe Y, Kiyosuke M. A dramatic increase in the positive blood culture rates of Helicobacter cinaedi The evidence of differential detection abilities between the Bactec and BacT/Alert systems. Diagn Microbiol Infect Dis. 2015. 83: 232-3
12. Morikawa M, Hirabayashi K, Akagawa D, Ishida T. A case of neonatal meningitis caused by Helicobacter cinaedi. J Jpn Soc Neonatal Health Dev. 2020. 32: 98-102
13. Narita E, Maruya J, Nishimaki K, Heianna J, Miyauchi T, Nakahata J. Case of infected subdural hematoma diagnosed by diffusion-weighted imaging. Brain Nerve. 2009. 61: 319-23
14. Okubo H, Goto M, Sato M, Sugiyama T, Kawano M, Matsunaga T. Helicobacter cinaedi meningitis: A case report and review of previous cases. J Neurol Sci. 2014. 347: 396-7
15. Orlicek SL, Welch DF, Kuhls TL. Septicemia and meningitis caused by Helicobacter cinaedi in a neonate. J Clin Microbiol. 1993. 31: 569-71
16. Oyama K, Khan S, Okamoto T, Fujii S, Ono K, Matsunaga T. Identification of and screening for human Helicobacter cinaedi infections and carriers via nested PCR. J Clin Microbiol. 2012. 50: 3893-900
17. Rampini SK, Bloemberg GV, Keller PM, Buchler AC, Dollenmaier G, Speck RF. Broad-range 16S rRNA gene polymerase chain reaction for diagnosis of culture-negative bacterial infections. Clin Infect Dis. 2011. 53: 1245-51
18. Sugiyama A, Mori M, Ishiwada N, Himuro K, Kuwabara S. First adult case of Helicobacter cinaedi meningitis. J Neurol Sci. 2014. 336: 263-4
19. Tamai S, Watanabe T, Ichinose T, Murakami KI, Ueno M, Munemoto S. Morphological characteristics of infected subdural hematoma: Comparison with images of chronic subdural hematoma. Clin Neurol Neurosurg. 2020. 194: 105831
20. Uwamino Y, Muranaka K, Hase R, Otsuka Y, Hosokawa N. Clinical features of community-acquired Helicobacter cinaedi bacteremia. Helicobacter. 2016. 21: 24-8