- Department of Neurosurgery, Sasebo City General Hospital, Sasebo, Nagasaki, Japan.
Correspondence Address:
Susumu Yamaguchi, Department of Neurosurgery, Sasebo City General Hospital, Sasebo, Nagasaki, Japan.
DOI:10.25259/SNI_955_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Susumu Yamaguchi, Michiharu Yoshida, Mitsuto Iwanaga. Hematoma expansion caused by trapped cerebrospinal fluid in subacute phase intracerebral hemorrhage: A case report. 11-Mar-2022;13:86
How to cite this URL: Susumu Yamaguchi, Michiharu Yoshida, Mitsuto Iwanaga. Hematoma expansion caused by trapped cerebrospinal fluid in subacute phase intracerebral hemorrhage: A case report. 11-Mar-2022;13:86. Available from: https://surgicalneurologyint.com/surgicalint-articles/11436/
Abstract
Background: Although hematoma expansion (HE) is caused by active bleeding in patients with intracranial hemorrhage in most cases, cerebrospinal fluid (CSF) trapped in the hematoma cavity is not a well-known cause of HE.
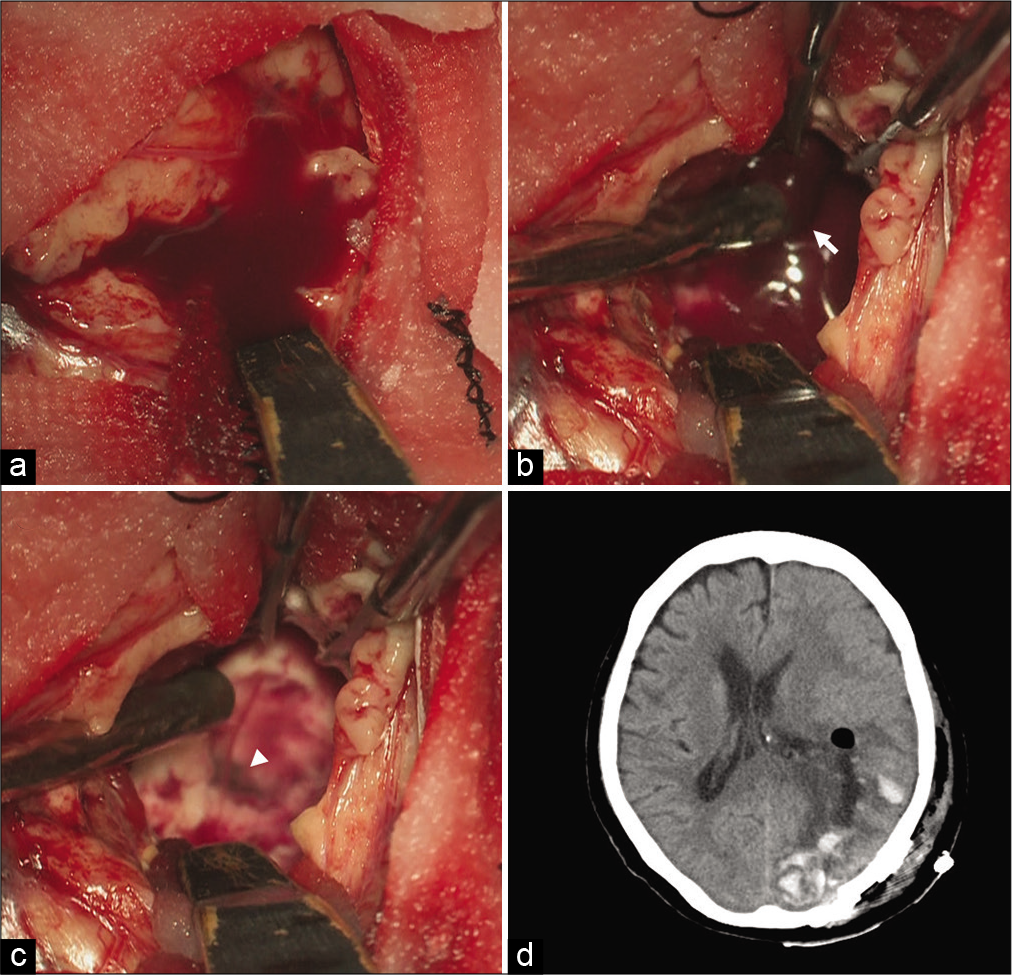
Case Description: We present a case of subcortical hemorrhage in an 80-year-old woman who experienced neurological deterioration in the subacute phase because of HE caused by CSF pooling in the hematoma cavity. The patient was transferred to our hospital from a previous hospital for surgical treatment because the consciousness disturbance was likely caused by the perihematomal edema that occurred 4 days after onset. Head computed tomography (CT) at admission to our hospital showed a blend sign, and a part of the low-density area of the hematoma was enlarged compared with the CT at admission to the previous hospital. Although the hematoma was located adjacent to the lateral ventricle, no intraventricular hemorrhage was observed. Emergent hematoma evacuation was performed, and intraoperative findings indicated that the enlarged hematoma cavity was caused by CSF pooling. The patient’s postoperative course was uneventful. She was transferred to a rehabilitation hospital 16 days after admission to our hospital.
Conclusion: Hematomas adjacent to the ventricle and showing a blend sign can expand in the subacute phase because of the trapped CSF.
Keywords: Blend sign, Cerebrospinal fluid, Hematoma expansion, Intracerebral hemorrhage, Perihematomal edema
INTRODUCTION
Hematoma expansion (HE) is independently associated with neurological deterioration in patients with intracranial hemorrhage (ICH) both in the hyperacute and acute phases.[
CASE DESCRIPTION
An 80-year-old woman with a history of cerebral infarction and hyperlipidemia complained of a sudden visual field defect and hemiparesis without disturbance of consciousness, and was hospitalized.
Head computed tomography (CT) performed 1.5 h from onset showed subcortical hemorrhage in the left occipital lobe. The patient received antihypertensive therapy. Four days after admission, her consciousness level decreased and she underwent a head CT. She was diagnosed with perihematomal edema and considered for hematoma evacuation and decompressive craniotomy. Subsequently, the patient was transferred to our hospital for further treatment. Neurological examination revealed consciousness disturbance, anisocoria, and right hemiparesis. She scored 5 points on the Glasgow Coma Scale (E1M3V1). Head CT during admission to our hospital [
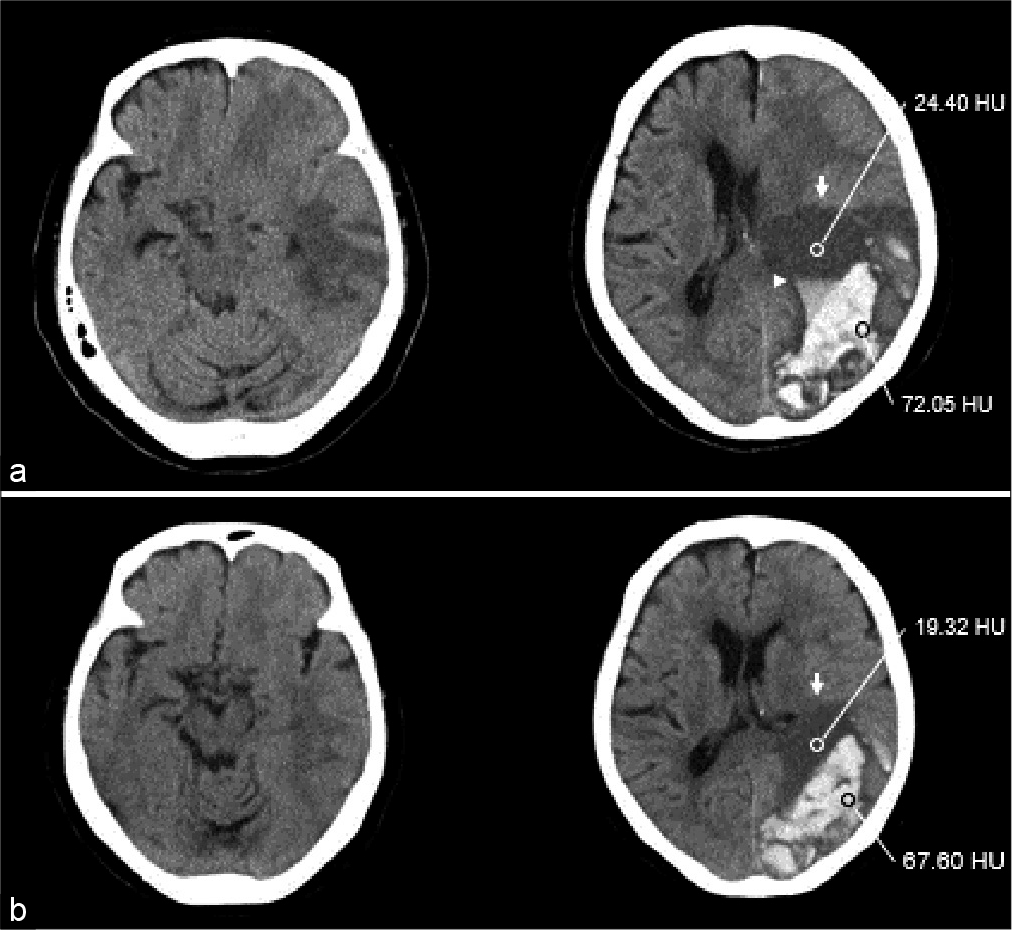
Figure 1:
(a) Computed tomography (CT) at admission to our hospital shows an enlargement of hematoma cavity with a worsened midline shift and uncal herniation. In spite of no hematoma growth, a part of the Low-density area (LDA) (arrow) with niveau formation (arrow head) in the hematoma cavity was enlarged. LDA and high-density area (HDA) CT values were 24.40 Hounsfield Unit (HU) and 72.05 HU, respectively. (b) CT at admission to a previous hospital shows subcortical hemorrhage at the occipitotemporal lobe with blend sign and no uncal herniation and intraventricular hemorrhage. LDA (arrow) and HDA CT values were 19.32 HU and 67.60 HU, respectively.
DISCUSSION
Herein, CT and intraoperative findings indicated that increased CSF components in the hematoma cavity led to HE and neurological deterioration. CSF, brain white matter, and hematoma CT values were 0–15 HU, <40 HU, and <80 HU, respectively.[
A CT blend sign is reportedly associated with HE. Indeed, in our case, CT performed on admission in the previous hospital showed a blend sign-like appearance: (1) blending of hypoattenuating and hyperattenuating regions with apparent margins between the two regions in the hematoma cavity; (2) >18 HU difference between the hypoattenuating and hyperattenuating regions in the hematoma; and (3) a hypoattenuating region not encapsulated in the hyperattenuating region.[
In general, HE caused by ICH occurs in the hyperacute phase. However, in cases of ICH adjacent to the ventricle, with a blend sign or trapped CSF, HE may occur in the subacute phase. Therefore, careful observation extending into the subacute phase is required, and the patients and their families need to be informed of this possibility. In addition, although we performed craniotomy hematoma evacuation with general anesthesia in the present case, stereotactic or endoscopic hematoma evacuation with local anesthesia, which are less invasive than craniotomy hematoma evacuation, might be considerable in ICH cases with trapped CSF as the main component of HE is estimated to be aspirable fluid-containing soft clot.
CONCLUSION
ICH cases with a CT blend sign are potential cases of hematoma with CSF, especially when the hematomas are adjacent to the ventricle without IVH. Careful observation is required, as in these cases, HE might often occur in the subacute phase.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ito H, Shimoji T, Yamamoto S, Saito K, Uehara S. Colloidal osmotic pressure in chronic subdural hematoma. Neurol Med Chir (Tokyo). 1988. 28: 650-3
2. Kim H, Kim GD, Yoon BC, Kim K, Kim BJ, Choi YH. Quantitative analysis of computed tomography images and early detection of cerebral edema for pediatric traumatic brain injury patients: Retrospective study. BMC Med. 2014. 12: 186
3. Li Q, Zhang G, Huang YJ, Dong MX, Lv FJ, Wei X. Blend sign on computed tomography: Novel and reliable predictor for early hematoma growth in patients with intracerebral hemorrhage. Stroke. 2015. 46: 2119-23
4. Lord AS, Gilmore E, Choi HA, Mayer SA. Time course and predictors of neurological deterioration after intracerebral hemorrhage. Stroke. 2015. 46: 647-52
5. Rózsa L, Grote EH, Egan P. Traumatic brain swelling studied by computerized tomography and densitometry. Neurosurg Rev. 1989. 12: 133-40