- Department of Neurological Surgery, Ryofukai Satoh Neurosurgical Hospital, Fukuyama,
- Department of Neurological Surgery, Okayama University Graduate School of Medicine, Okayama, Japan.
Correspondence Address:
Toru Satoh, Department of Neurological Surgery, Ryofukai Satoh Neurosurgical Hospital, Fukuyama, Japan.
DOI:10.25259/SNI_253_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Toru Satoh1, Yu Sato2, Kenji Sugiu2, Tomohito Hishikawa2, Masafumi Hiramatsu2, Jun Haruma2, Isao Date2. Hemifacial spasm due to vertebral artery dissecting aneurysm treated with stent-in-stent placement; Pre- and post-treatment evaluation by 3D multifusion imaging using silent MR angiography. 03-Jun-2022;13:232
How to cite this URL: Toru Satoh1, Yu Sato2, Kenji Sugiu2, Tomohito Hishikawa2, Masafumi Hiramatsu2, Jun Haruma2, Isao Date2. Hemifacial spasm due to vertebral artery dissecting aneurysm treated with stent-in-stent placement; Pre- and post-treatment evaluation by 3D multifusion imaging using silent MR angiography. 03-Jun-2022;13:232. Available from: https://surgicalneurologyint.com/surgicalint-articles/11635/
Abstract
Background: Hemifacial spasm (HFS) due to vertebral artery (VA) dissecting aneurysm (VADA) is rare and endovascular treatment has been performed in selected cases.
Case Description: We encountered a case of HFS caused by VADA that was managed with endovascular stent placement and additional stent-in-stent placement. Therapeutic strategies and benefits based on pre- and post-treatment evaluation by 3D multifusion imaging using silent MRA were discussed.
Conclusion: This is the first case report of stent-in-stent placement in successful treatment of HFS caused by VADA, in which relief of neurovascular contact was demonstrated by multifusion imaging.
Keywords: Endovascular treatment, Hemifacial spasm, Multifusion imaging, Stent-in-stent, VA dissecting aneurysm
INTRODUCTION
The incidence of hemifacial spasm (HFS) due to vertebral artery (VA) dissecting aneurysm (VADA) is as low as 0.2–0.5%.[
CASE DESCRIPTION
A 50s female suffered from the left HFS in 2012 and visited a nearby hospital. She received Botox treatment, but it was not effective. In 2014, she was referred to our hospital and diagnosed bilateral fusiform type VADAs by computed tomographic angiography [
Figure 2:
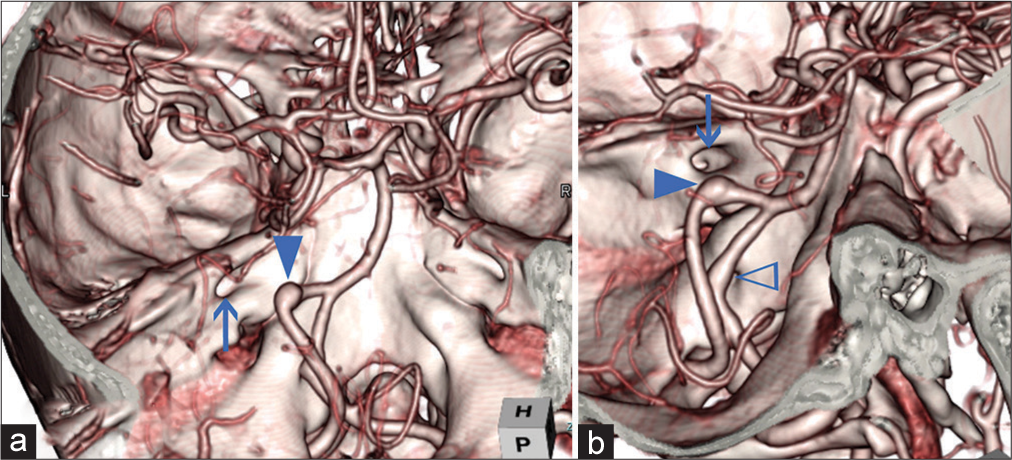
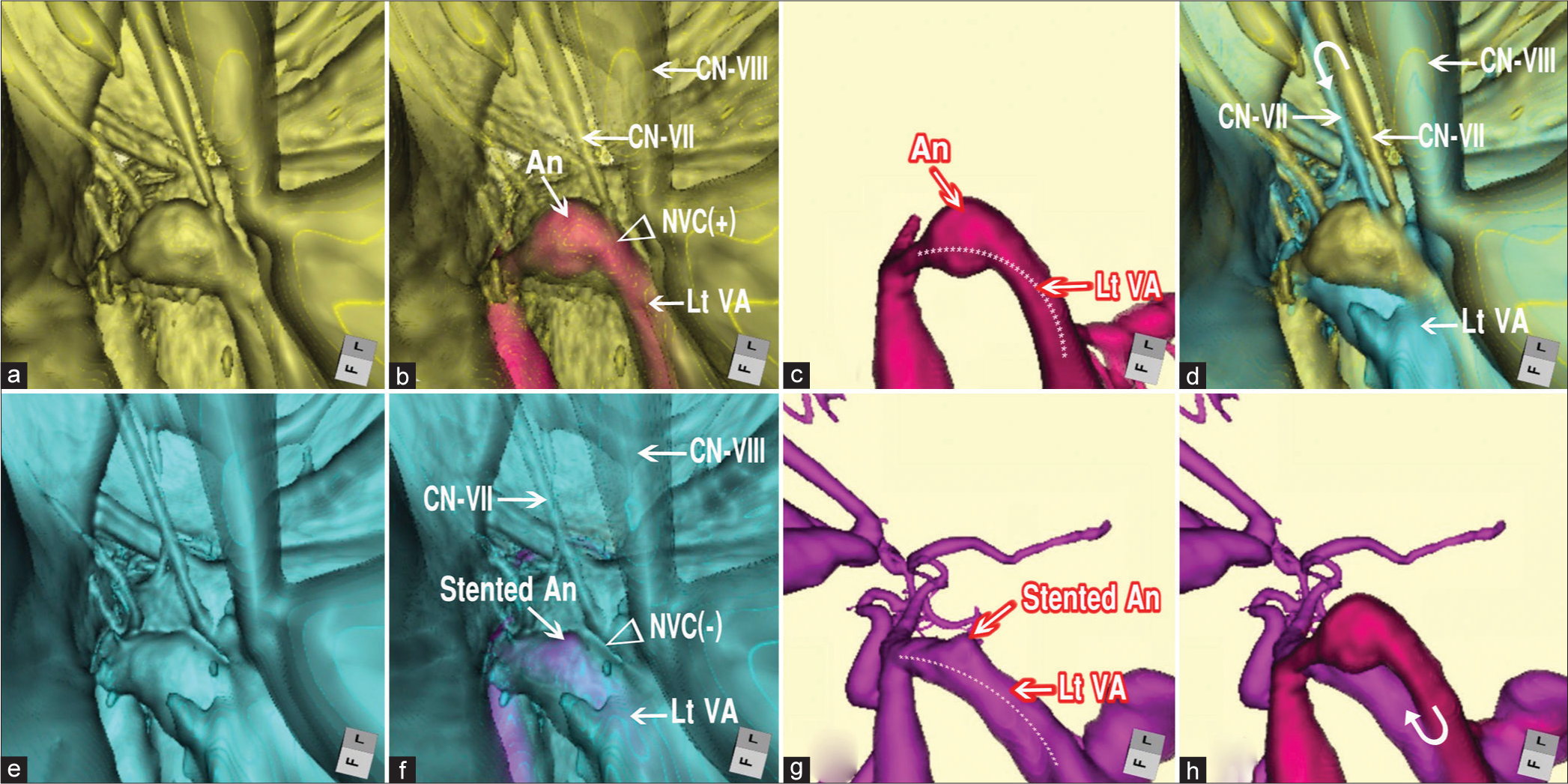
Pre- and post-treatment 3D multifusion images of HFS due to VADA. (a) Pretreatment 3D FSE MR cisternogram showing the NVC at the RExZ. The left facial nerve was directly compressed by the VADA. (b) Pretreatment 3D multifusion image of the time-of-flight MR angiography and fast spin-echo MR cisternography, same viewpoint as a. Arrowhead indicates the NVC. Arrows indicate CN-VII (facial nerve); CN-VIII, (vestibulocochlear nerve); VADA (An); VA. (c) Pretreatment 3D time-of-flight MR angiogram, same viewpoint as a, showing VADA. The dotted line indicates the running course of the left VA. (d) Pre- and post-treatment 3D multifusion image of the MR cisternogram, same viewpoint as a and e, showing the restoration form of the facial nerve after the second treatment through stentinstent placement. The curved arrow indicates the movement of the facial nerve. (e) Posttreatment 3D image of the MR cisternogram, same viewpoint as a, showing the elimination of NVC at the RExZ after the second treatment through stent-in-stent placement. (f) Posttreatment 3D multifusion image of silent MR angiogram and MR cisternogram, same viewpoint as a, showing the elimination of the NVC. (g) Posttreatment 3D silent MR angiogram, same viewpoint as a, showing the diminished size of the stented VADA. The dotted line indicates the running course of the left VA. (h) 3D multifusion images of (c) and (g). The curved arrow indicates the displacement of the left VA. HFS: Hemifacial spasm, MR: Magnetic resonance, VADA: Vertebral artery dissecting aneurysm, VA: VADA: Vertebral artery, RExZ: Root exit zone, NVC: Neurovascular contact.
Figure 4:
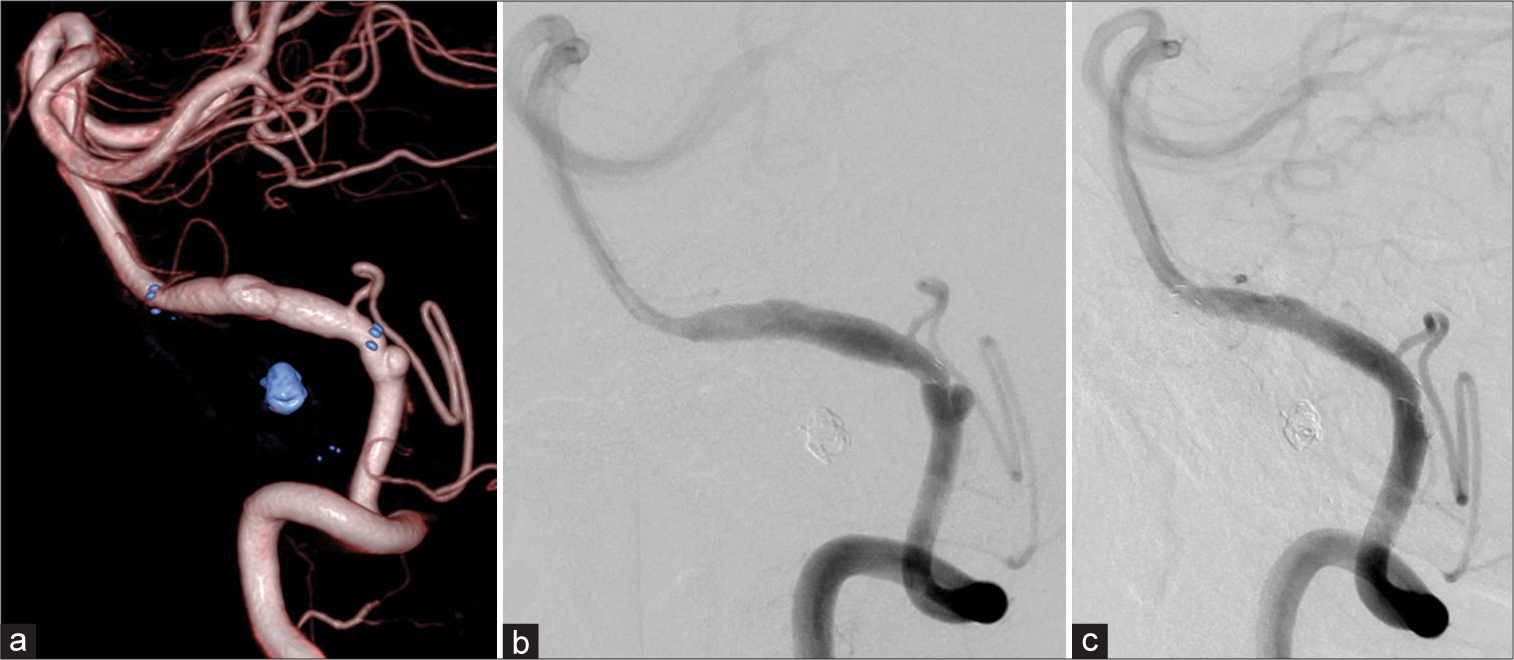
Digital angiography before the second treatment showing reduction of VADA, but appearance of a de novo aneurysm at the proximal end of the stent (a and b), after the second treatment using additional LVIS stent and coil (c). Note the running course of the parent VA was changed after the treatment. VADA: Vertebral artery dissecting aneurysm.
Figure 5:
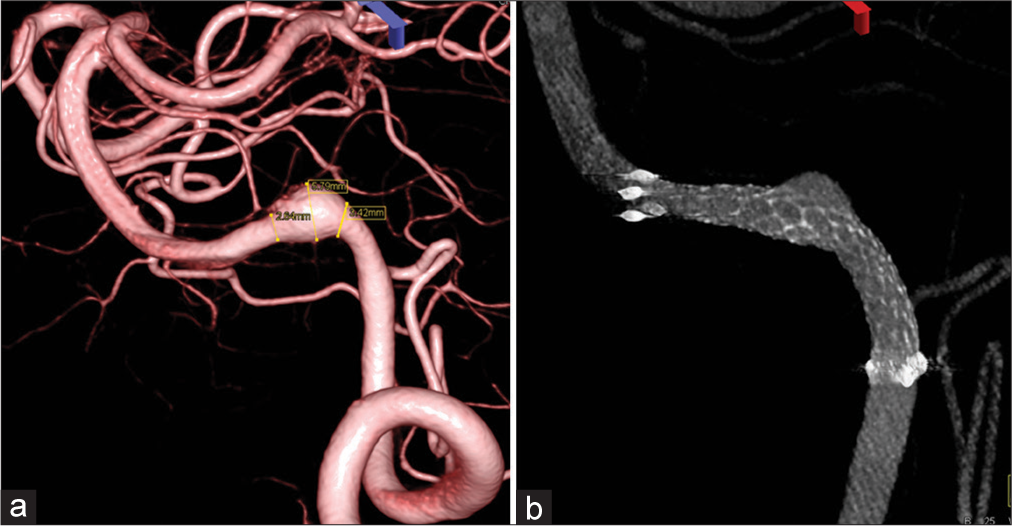
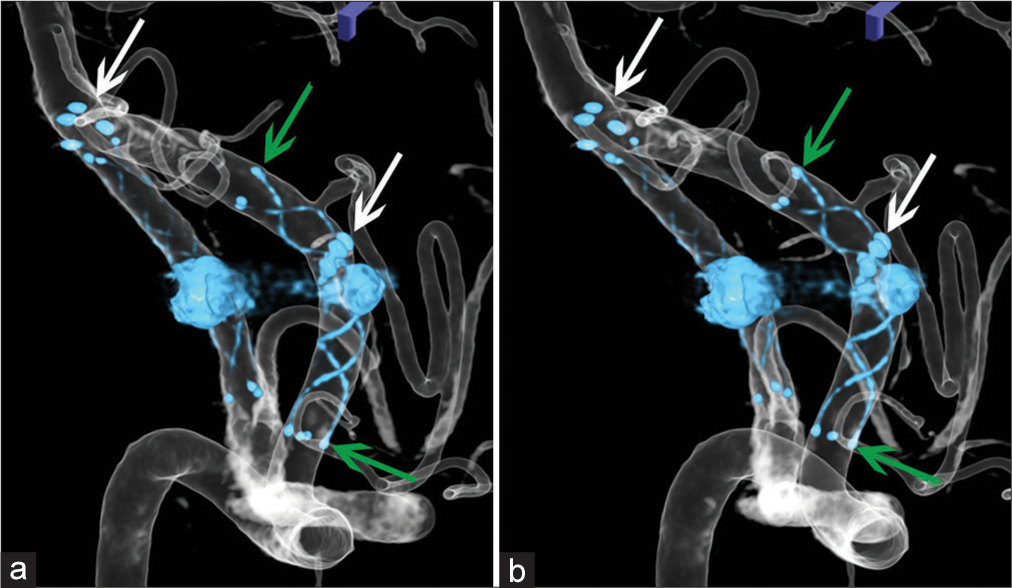
Stereotactic images (a and b) showing locations of two stents in the left VA after first and second treatments. White arrows indicate the proximal and distal ends of VRD (Enterprise 2 VRD 4/30) of the first treatment, and green arrows LVIS (additional stent-in-stent with LVIS-4.5/18) of the second treatment. VADA: Vertebral artery dissecting aneurysm.
DISCUSSION
Pretreatment image evaluation
HFS usually develops by NVC, in which the RExZ of the facial nerve is compressed by the responsible arteries such as the posterior inferior cerebellar artery, anterior inferior cerebellar artery, or VA.[
Endovascular treatment strategy for VADA causing HFS
Recently, endovascular treatments for VA aneurysm, such as aneurysmal coiling, internal trapping, stent-assisted coiling, and stent-in-stents, have been widely performed.[
Posttreatment image evaluation
Digital angiography has been performed for image evaluation after endovascular treatment of cerebral aneurysms with coils and stents. However, it is an invasive examination that involves risks such as procedural problems, side effects of iodine contrast media, and exposure to ionizing radiation. Therefore, time-of-flight MR angiography, with or without a contrast medium, is widely used as a noninvasive examination for long-term follow-up.[
In time-of-flight MR angiography, the vessel image is obtained by the flow void due to the maximum blood flow velocity during systole. The offending artery is depicted as the MR signal intensity due to the inflow effect of blood protons passing through the blood vessel. However, in the vicinity of the coils and stents, it is difficult to depict the morphology of the neck and parent arteries in detail because of the magnetic susceptibility artifact of the metal.[
Alternatively, silent MR angiography is a noncontrast and silent sound examination that uses an ultrashort TE (0.016 ms) with an artificial spin-labeling imaging sequence.[
In this case, silent MR angiography was performed for image evaluation after stent treatment. Posttreatment 3D multifusion imaging with silent MR angiography and MR cisternography visualized the shrinkage of VADA and disappearance of NVC [
Pre- and post-treatment image evaluation
The mechanism of endovascular treatment of VADA causing HFS has been considered to be because the coils and stents may reduce blood flow in the aneurysm and diminish arterial pulsation spread from the aneurysm wall over the facial nerve at the NVC site.[
In our case, the initial stent placement and additional stentin-stent placement reduced the size of the aneurysm. At the same time, the running pattern of the VA markedly changed, including VADA [
CONCLUSION
In the strategy of endovascular treatment for HFS due to VADA, stent and stent-in-stent placement was useful for diminishing the size of VADA by intra-aneurysmal thrombosis and for changes in the running course of the parent VA. Both may result in the elimination of NVC and complete cure of HFS. Relief of NVC was demonstrated by posttreatment 3D multifusion imaging with silent MR angiography and MR cisternography.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Ms. Megumi Sasaki, Ms. Kana Murakami, and Mr. Yudai Abe of our hospital for conducting the MRI examination.
References
1. Arisawa K, Ochi T, Goto Y, Nanbu S, Shojima M, Maeda K. Coil embolization of VA-PICA aneurysm presenting with hemifacial spasm with assistance of abnormal muscle response monitoring. J Neuroendovasc Ther. 2020. 14: 146-50
2. Iida Y, Mori K, Kawahara Y, Fukui I, Abe K, Takeda M. Hemifacial spasm caused by vertebral artery aneurysm treated by endovascular coil embolization. Surg Neurol Int. 2020. 11: 431
3. Kugai M, Suyama T, Inui T, Yamazato K, Kitano M, Hasegawa H. A case of vertebral artery aneurysm causing hemifacial spasm rapidly improved after parent artery occlusion. J Neuroendovasc Ther. 2019. 13: 288-92
4. Santiago-Dieppa DR, McDonald MA, Brandel MG, Rennert RC, Khalessi AA, Olson SE. Endovascular flow diversion for hemifacial spasm induced by a vertebral artery aneurysm: First experience. Oper Neurosurg (Hagerstown). 2019. 17: E115-8
5. Satoh T, Hishikawa T, Hiramatsu M, Sugiu K, Date I. Visualization of aneurysmal neck and dome after coiling with 3D multifusion imaging of silent MRA and FSE-MR cisternography. AJNR Am J Neuroradiol. 2019. 40: 802-7
6. Satoh T, Onoda K, Date I. Fusion imaging of three-dimensional magnetic resonance cisternograms and angiograms for the assessment of microvascular decompression in patients with hemifacial spasms. J Neurosurg. 2007. 106: 82-9
7. Sugiu K, Tokunaga K, Watanabe K, Sasahara W, Ono S, Tamiya T. Emergent endovascular treatment of ruptured vertebral artery dissecting aneurysms. Neuroradiology. 2005. 14: 158-64
8. Takano N, Suzuki M, Irie R, Yamamoto M, Hamasaki N, Kamagata K. Usefulness of non–contrast-enhanced MR angiography using a silent scan for follow-up after Y-configuration stent-assisted coil embolization for basilar tip aneurysms. AJNR Am J Neuroradiol. 2017. 38: 577-81
9. Wu X, Xu Y, Hong B, Zhao WY, Huang QH, Liu JM. Endovascular reconstruction for treatment of vertebrobasilar dolichoectasia. Long-term outcomes. AJNR Am J Neuroradiol. 2013. 34: 583-8
10. Yamashita K, Hojo M, Okamoto S, Kim C, Nakatsu S, Nishima H. Possible role of neurointerventional techniques in the diagnosis of hemifacial spasm. AJNR Am J Neuroradiol. 1997. 18: 287-90