- Department of Neurosurgery, Niigata University, Brain Research Institute, Niigata, Japan.
DOI:10.25259/SNI_780_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Tomoaki Suzuki, Hitoshi Hasegawa, Kazuhiro Ando, Kohei Shibuya, Haruhiko Takahashi, Shoji Saito, Jotaro On, Makoto Oishi, Yukihiko Fujii. Hemodynamic features of an intracranial aneurysm rupture predicted by perianeurysmal edema: A case report. 10-Feb-2021;12:49
How to cite this URL: Tomoaki Suzuki, Hitoshi Hasegawa, Kazuhiro Ando, Kohei Shibuya, Haruhiko Takahashi, Shoji Saito, Jotaro On, Makoto Oishi, Yukihiko Fujii. Hemodynamic features of an intracranial aneurysm rupture predicted by perianeurysmal edema: A case report. 10-Feb-2021;12:49. Available from: https://surgicalneurologyint.com/surgicalint-articles/10583/
Abstract
Background: Perianeurysmal edema (PAE) has been suggested as an indicator of potential aneurysm rupture; however, the hemodynamic features of these aneurysms are still unknown. A computational fluid dynamic (CFD) analysis was performed to evaluate the hemodynamic features of a very rare case of a ruptured middle cerebral artery (MCA) aneurysm with PAE.
Case Description: A 65-year-old woman presented with disturbed consciousness. A subarachnoid hemorrhage due to an azygos anterior cerebral artery (ACA) aneurysm rupture was suspected. An unruptured MCA aneurysm with PAE was identified in the left temporal lobe. Although the ACA aneurysm was clipped to prevent re-bleeding, the MCA aneurysm subsequently ruptured 6 days later. Clipping of the MCA aneurysm was performed, and hemosiderin deposits suggestive of sentinel bleeding were found on the surface of the aneurysm dome. CFD analysis revealed unstable hemodynamic stress at the expanded bleb area after rupture, localized to the rupture site. Moreover, this analysis revealed flow impingement with pressure elevation and low wall shear stress, which indicated increased inflammation and aneurysm wall thinning that likely led to rupture.
Conclusion: Hemosiderin deposits at the aneurysm wall and PAE indicates leakage from a cerebral aneurysm. Hemodynamic stress at the aneurysm may promote an inflammatory response and lead to wall weakening accompanied by PAE. Based on our findings, we recommend that surgical intervention should be considered as the first line of treatment for such aneurysms to prevent rupture.
Keywords: Aneurysm rupture, Case report, Computational fluid dynamics, Hemosiderin deposition, Perianeurysmal edema
INTRODUCTION
Subarachnoid hemorrhage (SAH) after an intracranial aneurysm rupture results in high mortality and morbidity. Structural fragility of the aneurysm wall-induced inflammatory response is associated with abnormal hemodynamics and an increased likelihood of aneurysm rupture.[
CASE DESCRIPTION
This study was approved by the Institutional Ethics Committee at our Hospital (Approval number: 2020-0027). Informed consent was obtained from the patient’s guardian.
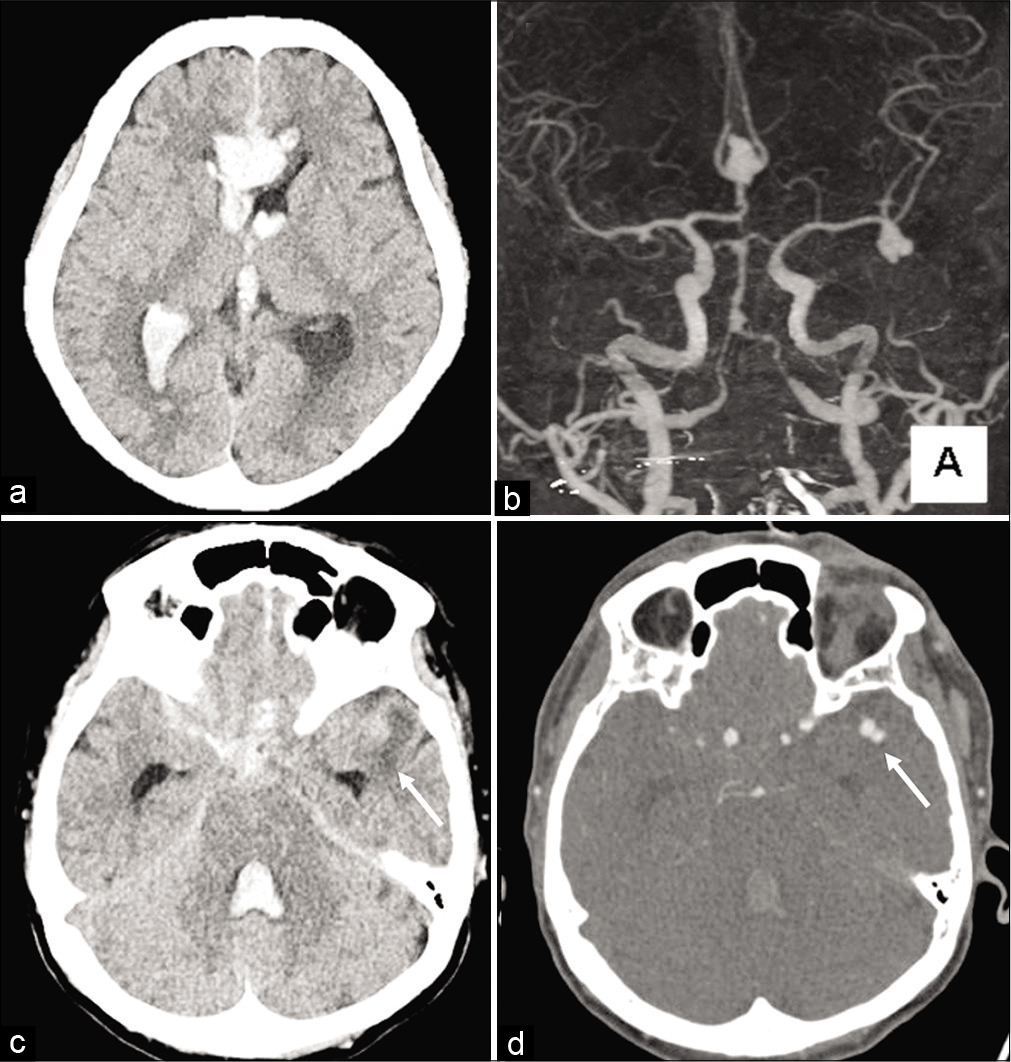
A 65-year-old woman was admitted to our hospital with disturbed consciousness. She had a medical history of hypertension, but no history of smoking or alcohol consumption. Computed tomography (CT) revealed a thick SAH in the interhemispheric fissure and an intracerebral hemorrhage (ICH) in the frontal lobe [
Figure 1:
Initial computed tomography (CT) image showing thick subarachnoid hemorrhage in the interhemispheric fissure and intracerebral hemorrhage in the frontal lobe (a). CT angiography demonstrating both an azygos anterior cerebral artery (ACA) aneurysm and a left middle cerebral artery (MCA) aneurysm. Based on the hemorrhage distribution, the azygos ACA aneurysm was suspected to be ruptured (b). Perianeurysmal edema around the bleb of the MCA aneurysm was detected in the left temporal lobe (c and d; white arrow).
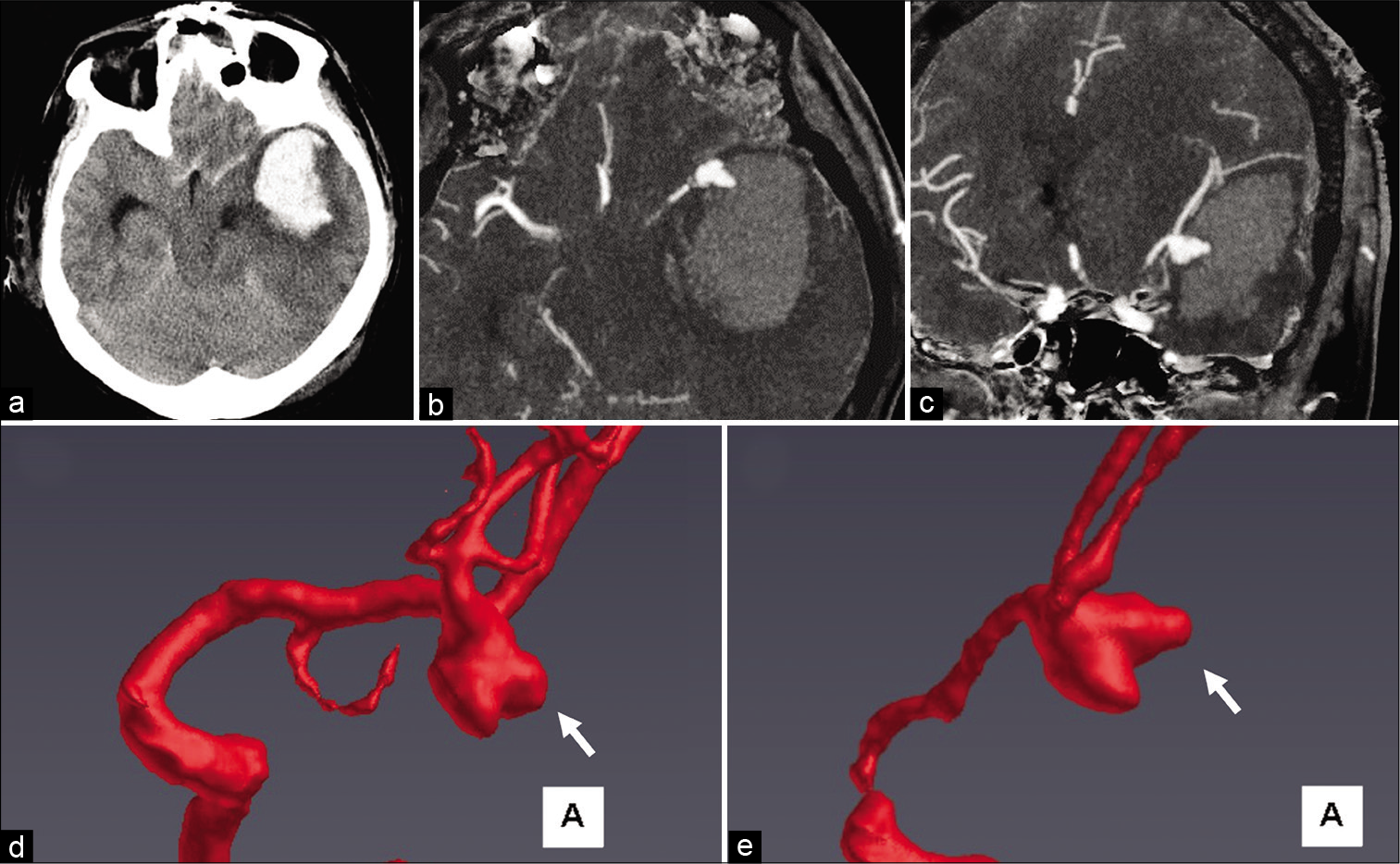
Figure 2:
Computed tomography (CT) image showing a massive intracerebral hemorrhage in the left temporal lobe (a). During CT angiography (CTA), the suspected rupture point was identified at the expanded bleb (b) axial view, and (c) coronal view. Morphological changes in the bleb (white arrow) were detected in 3D CTA images before and after rupture (d) before rupture and (e) after rupture.
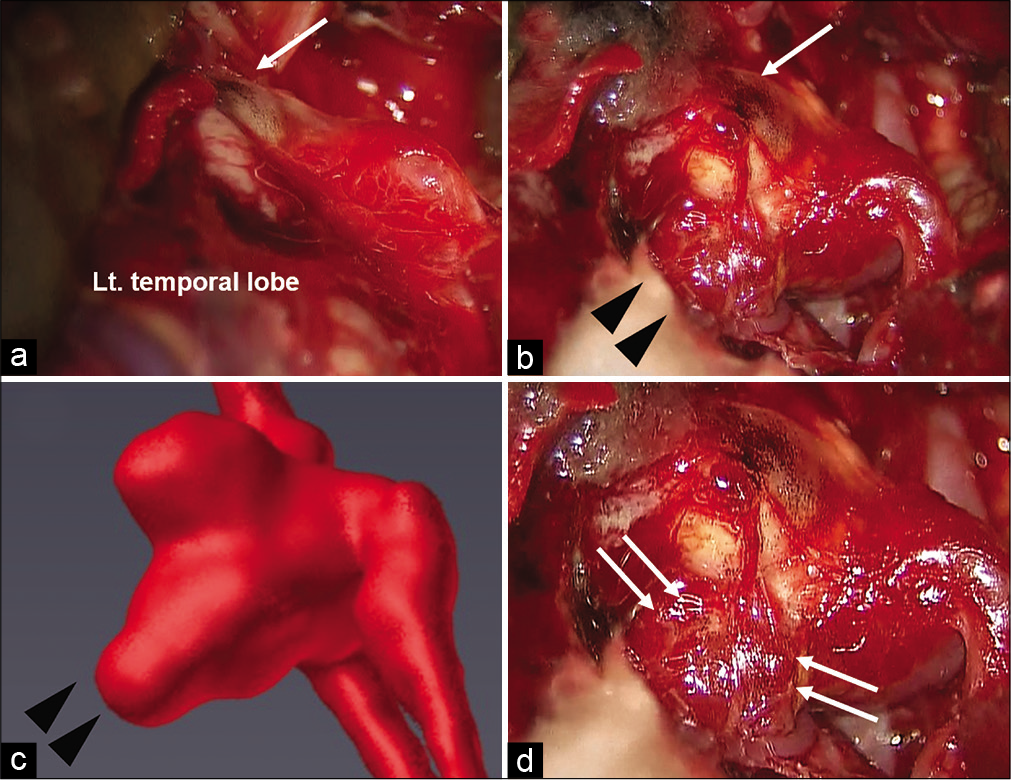
Figure 3:
Intraoperative photographs. The left middle cerebral artery aneurysm was buried in the left temporal lobe, and hemosiderin deposition was observed around the aneurysm fundus (a; white arrows). The rupture point was detected in the thinning and expanded bleb (b; black arrow heads). The same intraoperative view of the 3D computed tomography angiography after rupture (c), with black arrow heads indicating the expanded bleb at the rupture point (c). Hemosiderin deposition was also observed surrounding the rupture point (d; white arrows).
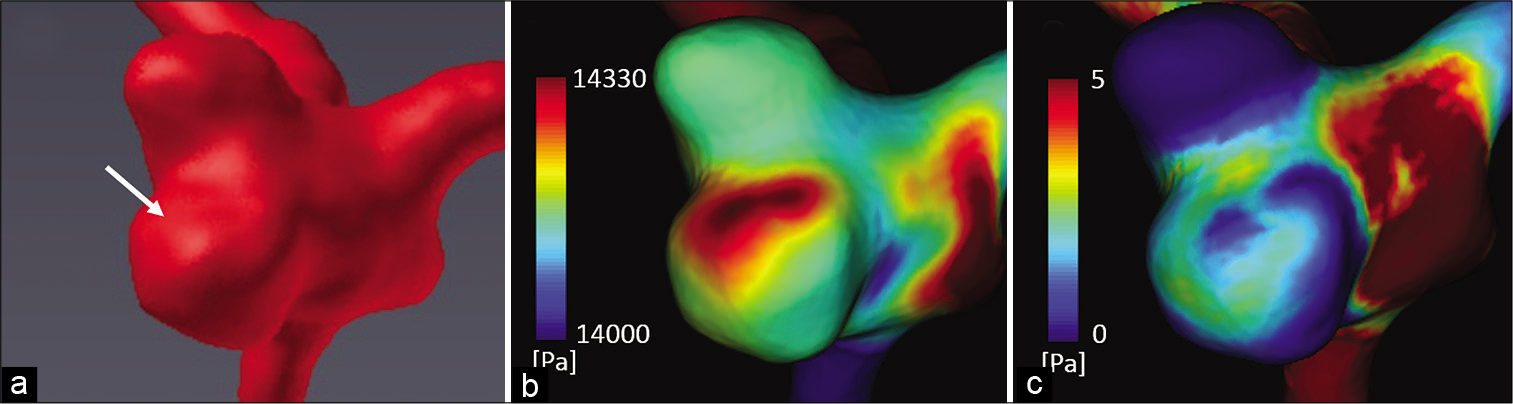
Figure 4:
3D computed tomography angiography of the operative view before rupture (a) and computational fluid dynamic analysis results (b and c). In the region of the expanded bleb after rupture (a; white arrow), pressure elevation with maximum pressure at the peak systolic phase (b), and low wall shear stress at the end diastolic phase (c) were clearly observed.
DISCUSSION
This is the first report to establish the intraoperative observations of hemosiderin deposition, PAE, and abnormal hemodynamic stress (detected via CFD) at the aneurysm dome as warning signs of aneurysm rupture. This study had some limitations: Only one case of MCA aneurysm was analyzed, and hemosiderin deposition and aneurysm wall integrity were only intraoperatively evaluated and not pathologically.
Brain edema is likely to be observed around large, partially thrombosed aneurysms and is often detected after coil embolization.[
This is consistent with the theory that low WSS diminishes endothelial cell integrity and weakens the aneurysm wall.[
This finding underscores the need to consider the early treatment of aneurysms with PAE. This is especially important when considering further decisions after another aneurysm rupture, as some drugs for cerebral vasospasm could promote dangerous bleeding. CFD analysis is useful for identifying hemodynamic instability that may eventually lead to aneurysm rupture. We report that combining a PAE evaluation with CFD analysis is a promising tool for assessing the risk of aneurysm rupture and can help guide preventative interventions.
CONCLUSION
Our case demonstrates that hemosiderin deposition on the aneurysm wall with PAE indicates minor leakage of the aneurysm and impending rupture. The CFD analysis suggested that unstable hemodynamic stress (i.e., pressure elevation: flow impingement and low WSS) may promote aneurysm wall inflammation and weaken the wall, accompanied by PAE. In such cases, our findings indicate that preventative surgical interventions may be warranted to avoid rupture.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
SUPPLEMENTAL DIGITAL CONTENT
Supplemental methods: CFD analysis using hemoscope v1.4
Hemoscope v1.4 (EBM Corp, Tokyo, Japan) was used for the CFD analysis. This commercially available tool allowed us to study blood flow in cerebral aneurysms. Vascular geometry was obtained from Digital Imaging and Communications in Medicine data from CTA images and was reconstructed using a medical image processing package (Ziostation2; Ziosoft, Inc., Japan). The present computation adopted a pulsatile flow rate, and blood flow along the computational mesh processing was simulated with Navier-Stokes equations. Physical properties of blood were set as an incompressible Newtonian fluid with a density of 1050 kg/m3 and a dynamic viscosity of 0.004 Pa•s. Rigid blood vessel walls were assumed in the simulation. The total flow rate was based on a uniform WSS hypothesis and a constant value of τ= 1.5 Pa was used.
The boundary conditions were determined in accordance with a constant wall shear stress theory; the flow rates of the inlet and outlet vessels were calculated using the following equation:
where Q, τ, μ, and D denote the flow rate, wall shear stress, fluid viscosity, and vascular diameter, respectively. The equation is a well-known theoretical basis of a fully developed laminar pipe flow. Herein, the WSS was set at τ= 1.5 Pa. Inlet pressure was set as 100 mmHg and flow distribution for each outlet was determined according to Murray’s law.
References
1. Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH. Biology of intracranial aneurysms: Role of inflammation. J Cereb Blood Flow Metab. 2012. 32: 1659-76
2. Dengler J, Maldaner N, Bijlenga P, Burkhardt JK, Graewe A, Guhl S. Perianeurysmal edema in giant intracranial aneurysms in relation to aneurysm location, size, and partial thrombosis. J Neurosurg. 2015. 123: 446-52
3. Frösen J, Cebral J, Robertson AM, Aoki T. Flow-induced, inflammation-mediated arterial wall remodeling in the formation and progression of intracranial aneurysms. Neurosurg Focus. 2019. 47: E21
4. Hiu T, Tsutsumi K, Kitagawa N, Hayashi K, Ujifuku K, Yasunaga A. Progressive perianeurysmal edema preceding the rupture of a small basilar artery aneurysm. Clin Neurol Neurosurg. 2009. 111: 216-9
5. Kataoka K, Taneda M, Asai T, Kinoshita A, Ito M, Kuroda R. Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke. 1999. 30: 1396-401
6. Murayama Y, Fujimura S, Suzuki T, Takao H. Computational fluid dynamics as a risk assessment tool for aneurysm rupture. Neurosurg Focus. 2019. 47: E12
7. Nussbaum ES, Defillo A, Zelensky A, Pulivarthi S, Nussbaum L. “Microbleeding” from intracranial aneurysms: Local hemosiderin deposition identified during microsurgical treatment of unruptured intracranial aneurysms. Surg Neurol Int. 2014. 5: 28
8. Pahl FH, de Oliveira MF, Ferreira NP, de Macedo LL, Brock RS, de Souza VC. Perianeurysmal edema as a predictive sign of aneurysmal rupture. J Neurosurg. 2014. 121: 1112-4
9. Sejkorová A, Dennis KD, Švihlová H, Petr O, Lanzino G, Hejčl A. Hemodynamic changes in a middle cerebral artery aneurysm at follow-up times before and after its rupture: A case report and a review of the literature. Neurosurg Rev. 2017. 40: 329-38
10. Su IC, Willinsky RA, Fanning NF, Agid R. Aneurysmal wall enhancement and perianeurysmal edema after endovascular treatment of unruptured cerebral aneurysms. Neuroradiology. 2014. 56: 487-95
11. Sugiyama S, Niizuma K, Sato K, Rashad S, Kohama M, Endo H. Blood flow into basilar tip aneurysms: A predictor for recanalization after coil embolization. Stroke. 2016. 47: 2541-7
12. Suzuki T, Stapleton CJ, Koch MJ, Tanaka K, Fujimura S, Suzuki T. Decreased wall shear stress at high-pressure areas predicts the rupture point in ruptured intracranial aneurysms. J Neurosurg. 2019. 132: 1116-22
13. Suzuki T, Takao H, Suzuki T, Kambayashi Y, Watanabe M, Sakamoto H. Determining the presence of thin-walled regions at high-pressure areas in unruptured cerebral aneurysms by using computational fluid dynamics. Neurosurgery. 2016. 79: 589-95
14. Tulamo R, Frösen J, Hernesniemi J, Niemelä M. Inflammatory changes in the aneurysm wall: A review. J Neurointerv Surg. 2010. 2: 120-30
15. Turjman AS, Turjman F, Edelman ER. Role of fluid dynamics and inflammation in intracranial aneurysm formation. Circulation. 2014. 129: 373-82