- Department of Neurological Surgery, Wakayama Medical University, Wakayama, Japan.
Correspondence Address:
Takahiro Sasaki, Department of Neurological Surgery, Wakayama Medical University, Wakayama, Japan.
DOI:10.25259/SNI_1130_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kazuhide Maeshima, Takahiro Sasaki, Toshikazu Yamoto, Junya Fukai, Hiroki Nishibayashi, Naoyuki Nakao. Hemorrhagic brain metastasis from small-cell carcinoma of the urinary bladder. 20-Jan-2022;13:20
How to cite this URL: Kazuhide Maeshima, Takahiro Sasaki, Toshikazu Yamoto, Junya Fukai, Hiroki Nishibayashi, Naoyuki Nakao. Hemorrhagic brain metastasis from small-cell carcinoma of the urinary bladder. 20-Jan-2022;13:20. Available from: https://surgicalneurologyint.com/surgicalint-articles/11356/
Abstract
Background: Small-cell carcinoma of the urinary bladder (SCCB) accounts for 1% of all bladder tumors. We present a rare case of hemorrhagic metastatic brain tumor from SCCB diagnosed by navigation-guided endoscopic biopsy.
Case Description: A 76-year-old man presented with sudden onset of aphasia and right hemiplegia from 3 weeks previously. He had a medical history of prostate cancer and SCCB. Computed tomography showed a mixed density mass in the left basal ganglia. On magnetic resonance imaging, the mass showed mixed intensity in both T1-weighted images and T2-weighted images, suggesting subacute hemorrhage. The mass was partially enhanced with gadolinium. The patient underwent endoscopic hematoma evacuation and partial removal of the tumor. Histopathological diagnosis was neuroendocrine carcinoma, which was consistent with SCCB metastasis. After surgery, the patient underwent whole-brain radiation therapy of 30 Gy. His general condition gradually deteriorated, however, and he died 4 months after surgery.
Conclusion: Our patient had a rare case of brain metastasis derived from SCCB which presented with cerebral hemorrhage. Navigation-guided endoscopic biopsy was useful for the diagnostic sampling of deep localized brain tumors with hemorrhage.
Keywords: Hemorrhagic brain metastasis, Navigation-guided endoscopic biopsy, Small-cell carcinoma of the urinary bladder
INTRODUCTION
Small-cell carcinoma of the urinary bladder (SCCB) is a very rare neuroendocrine tumor, accounting for only about 1% of all bladder cancers.[
CASE DESCRIPTION
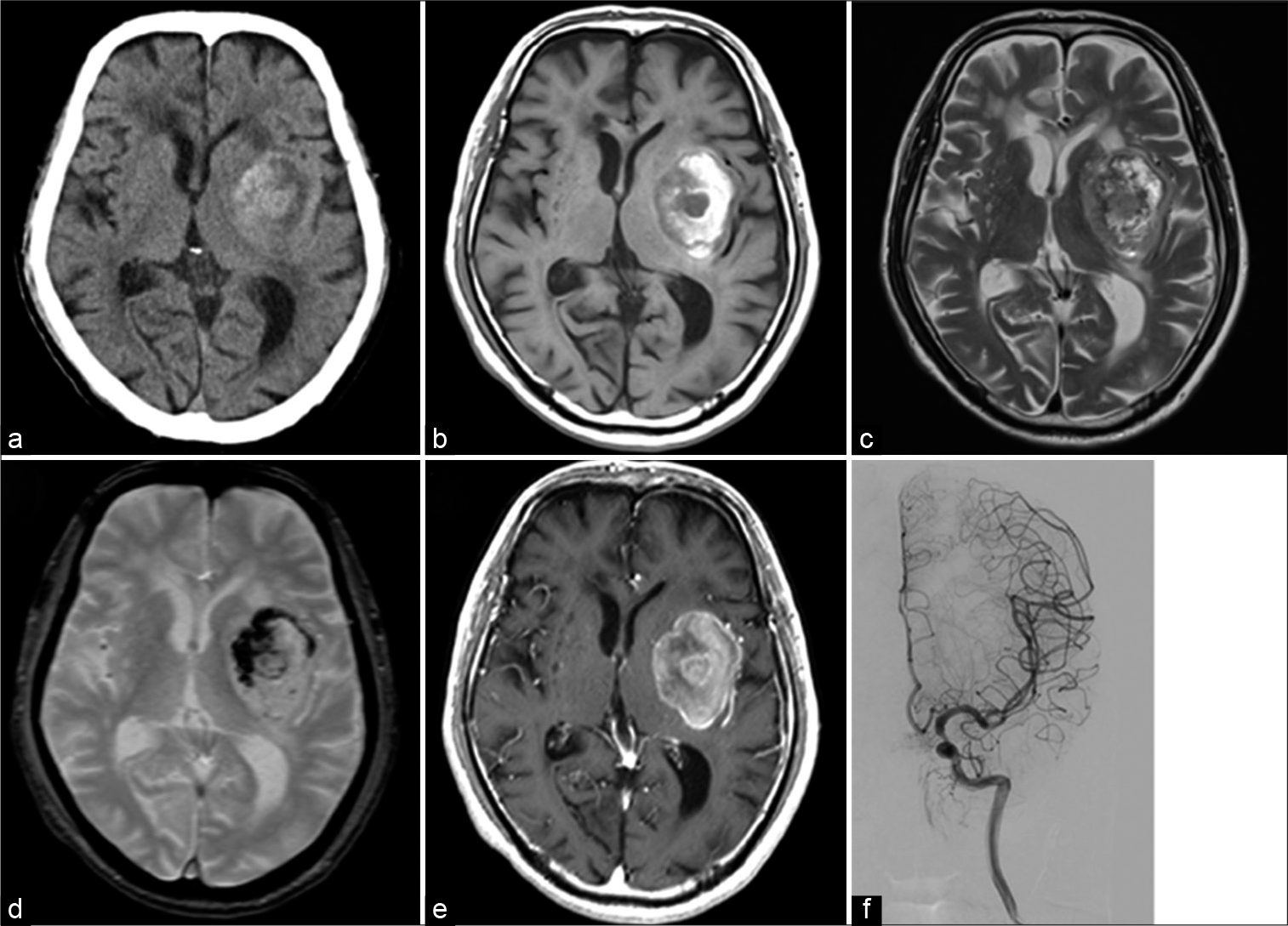
A 76-year-old man was referred to our outpatient department with the complaints of sudden onset of aphasia and right hemiplegia from a few weeks previously. He underwent transurethral resection of SCCB 2 years before the current presentation. After three courses of preoperative chemotherapy with irinotecan and carboplatin, the patient underwent total cystectomy. After the surgery, no local or systemic metastasis was observed in periodic positron emission tomography. He had not been examined with brain magnetic resonance imaging (MRI) for screening after total cystectomy. On admission, Glasgow Coma Scale was 14 (E4V4M6), and physical examination revealed motor aphasia and right hemiparalysis (manual muscle test, 3/5). Head computed tomography (CT) showed a 40 mm diameter round, mixed-density lesion in the left basal ganglia [
Figure 1:
Preoperative computed tomography scan (a) shows a mixed density mass in the left basal ganglia. On magnetic resonance imaging, the mass shows mixed intensity in T1-weighted images (b), T2-weighted images (c), and T2*-weighted images (d), suggesting subacute hemorrhage. The mass is partially enhanced with gadolinium (e), cerebral angiography (f) shows no tumor stains.
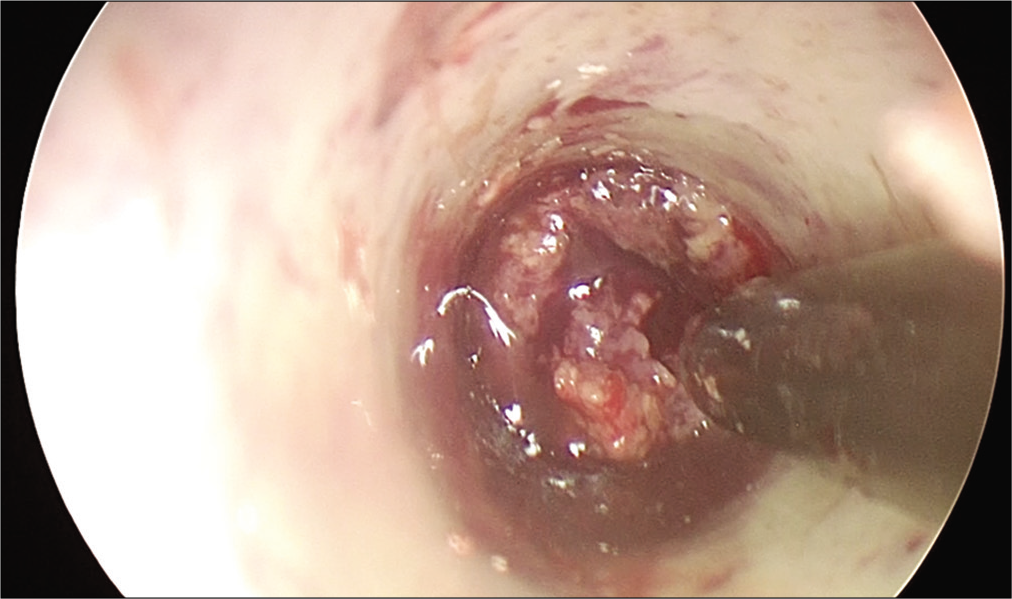
Hematoma evacuation and partial tumor removal were performed by navigation-guided endoscopy. After removal of the hematoma, the solid tumor was partially removed [
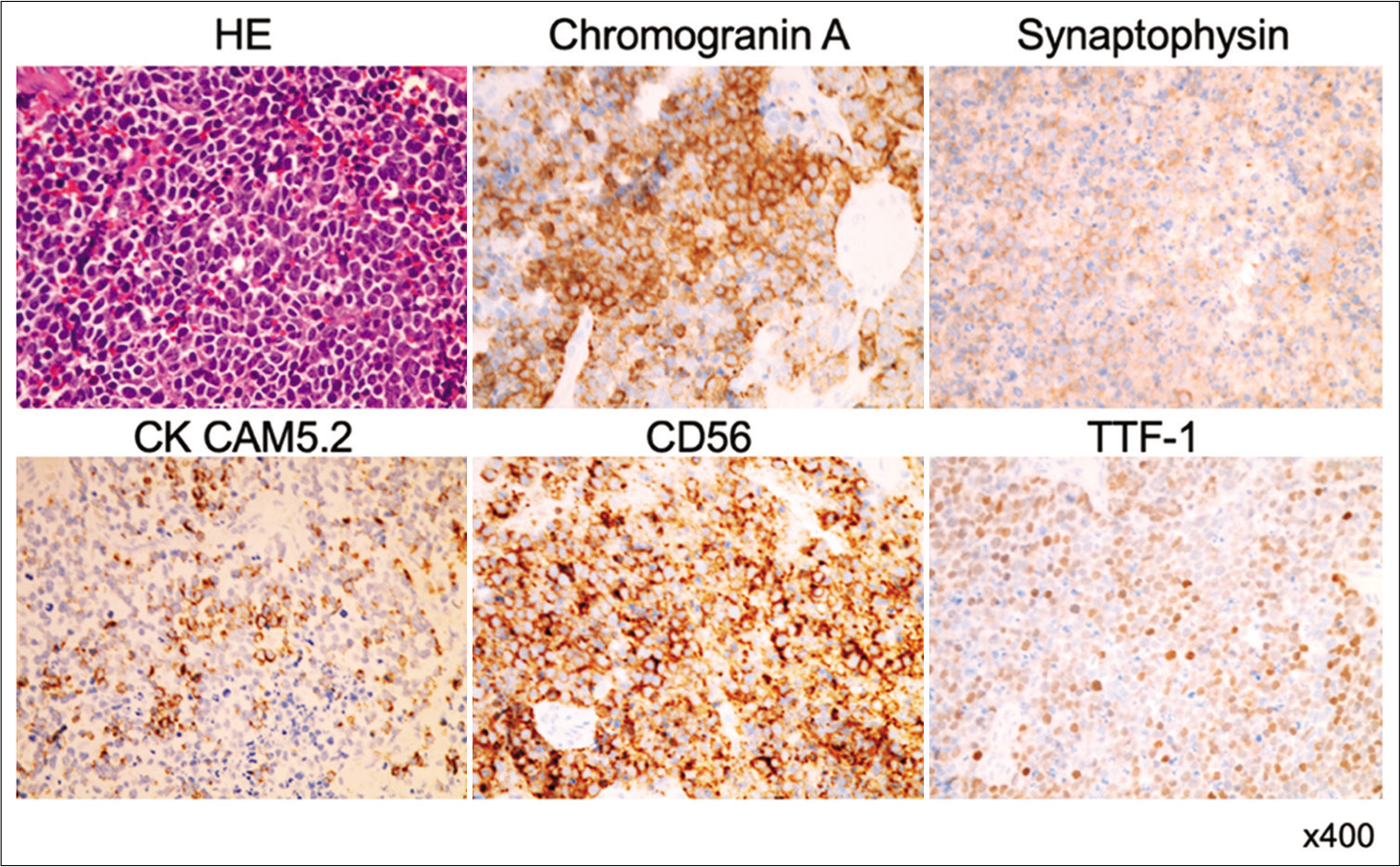
Hematoxylin and eosin staining showed diffuse sheets of small atypical cells with blood vessels [
Figure 4:
Photomicrographs of the surgical specimen stained with hematoxylin and eosin show dense sheets of malignant small round cells with hyperchromatic nuclei. Immunohistochemical stains show that tumor cells are immunoreacted for synaptophysin, chromogranin A, CK CAM5.2, CD56, and thyroid transcription factor 1.
His aphasia and right hemiplegia did not improve after the surgery. Whole-body scintigraphy showed no other metastases. The patient was diagnosed with single-organ metastasis to the brain and he underwent whole-brain irradiation (30 Gy/10 fraction), but no additional chemotherapy was administered in consideration of his low Karnofsky performance status (40 scores). The patient was transferred to a rehabilitation hospital, but his general condition gradually deteriorated and he died 4 months after surgery.
DISCUSSION
SCCB is a type of neuroendocrine carcinoma with similar histopathological features to that of small-cell carcinoma of the lung. It accounts for 1% of all bladder tumors, with a strong male predominance.[
Due to its low disease incidence, the optimal therapeutic strategy of SCCB remains uncertain. Neoadjuvant chemotherapy, followed by surgical resection and adjuvant chemotherapy, has shown better outcomes in retrospective studies.[
Intracerebral hemorrhage (ICH) derived from brain metastasis was present in 14% of all reported cases of brain metastasis.[
At the subacute stage of cerebral hemorrhage, the surrounding blood–brain barrier is disrupted by the hemorrhage, and neovascularization without blood–brain barrier causes contrast enhancement in the hemorrhage site and its surrounding area.[
CONCLUSION
Our patient presented a rare case of hemorrhagic brain metastasis of SCCB. Navigation-guided endoscopic biopsy was useful for the diagnostic sampling of intraparenchymal brain tumors with hemorrhage.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We acknowledge proofreading and editing by Benjamin Phillis at the clinical study support at Wakayama Medical University.
References
1. Aksoy D, Bammer R, Mlynash M, Venkatasubramanian C, Eyngorn I, Snider RW. Magnetic resonance imaging profile of blood-brain barrier injury in patients with acute intracerebral hemorrhage. J Am Heart Assoc. 2013. 2: e000161
2. Bex A, Sonke GS, Pos FJ, Brandsma D, Kerst JM, Horenblas S. Symptomatic brain metastases from small-cell carcinoma of the urinary bladder: The Netherlands cancer institute experience and literature review. Ann Oncol. 2010. 21: 2240-5
3. Choong NW, Quevedo JF, Kaur JS. Small cell carcinoma of the urinary bladder: The mayo clinic experience. Cancer. 2005. 103: 1172-8
4. Isaka T, Maruno M, Sato M, Kinoshita M, Nishida T, Kiyohara H. Brain metastasis from small-cell neuroendocrine carcinoma of the urinary bladder. Brain Tumor Pathol. 2002. 19: 117-22
5. Ishikawa E, Yamamoto T, Matsuda M, Akutsu H, Zaboronok A, Kohzuki H. Intraparenchymal brain-lesion biopsy guided by a rigid endoscope and navigation system. Surg Neurol Int. 2015. 6: 149
6. Ismaili N. A rare bladder cancer-small cell carcinoma: Review and update. Orphanet J Rare Dis. 2011. 6: 75
7. Keep RF, Zhou N, Xiang J, Andjelkovic AV, Hua Y, Xi G. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS. 2014. 11: 18
8. Lynch SP, Shen Y, Kamat A, Grossman HB, Shah JB, Millikan RE. Neoadjuvant chemotherapy in small cell urothelial cancer improves pathologic downstaging and long-term outcomes: Results from a retrospective study at the MD Anderson cancer center. Eur Urol. 2013. 64: 307-13
9. Ma R, Fang SK, Hou S, Wang X, Meng HM. Suspected brain metastasis from lung cancer mimicking intracerebral hemorrhage: A case report. Medicine (Baltimore). 2018. 97: 2-4
10. Tsuda K, Ishikawa E, Zaboronok A, Nakai K, Yamamoto T, Sakamoto N. Navigation-guided endoscopic biopsy for intraparenchymal brain tumor. Neurol Med Chir (Tokyo). 2011. 51: 694-700
11. Warrick JI. Clinical significance of histologic variants of bladder cancer. J Natl Compr Canc Netw. 2017. 15: 1268-74
12. Yoo H, Jung E, Gwak HS, Shin SH, Lee SH. Surgical outcomes of hemorrhagic metastatic brain tumors. Cancer Res Treat. 2011. 43: 102-7
13. Zhao X, Flynn EA. Small cell carcinoma of the urinary bladder a rare, aggressive neuroendocrine malignancy. Arch Pathol Lab Med. 2012. 136: 1451-9