- Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, Tennessee,
- Department of Neuroscience, Centre for Neuroscience, Queens University, Kingston, Ontario, Canada,
- Department of Neurosurgery, University at Buffalo, 40 George Karl Blvd, Williamsville, Buffalo, New York, United States,
- Section of Neurosurgery, Aga Khan University Hospital, Karachi, Sindh, Pakistan.
- Department of Surgery, Aga Khan University Hospital, Karachi, Sindh, Pakistan.
Correspondence Address:
Muhammad Shahzad Shamim
Department of Surgery, Aga Khan University Hospital, Karachi, Sindh, Pakistan.
DOI:10.25259/SNI_607_2019
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Inamullah Khan1, Ayesha Quddusi2, Muhammad Waqas3, Hamid Hussain Rai3, Saqib Kamran Bakhshi4, Muhammad Shahzad Shamim5, Rashid Jooma5. Hemorrhagic complications after decompressive craniectomy. 11-Nov-2020;11:379
How to cite this URL: Inamullah Khan1, Ayesha Quddusi2, Muhammad Waqas3, Hamid Hussain Rai3, Saqib Kamran Bakhshi4, Muhammad Shahzad Shamim5, Rashid Jooma5. Hemorrhagic complications after decompressive craniectomy. 11-Nov-2020;11:379. Available from: https://surgicalneurologyint.com/surgicalint-articles/10393/
Abstract
Background: Decompressive craniectomy (DC) is the preferred surgical management option for lowering refractory intracranial pressure in cases of traumatic brain injury (TBI). A number of randomized controlled trials have demonstrated decreased mortality but increased morbidity following DC for TBI patients. Here, we reviewed the frequency of postoperative hemorrhagic complications following DC correlating with poor outcomes.
Methods: We retrospectively reviewed the medical records of patients who presented with TBI and underwent DC during the years 2015–2017. The frequency and characteristics of hemorrhagic complications were correlated with the patients’ outcomes.
Results: There were 74 patients with TBI included in the study who underwent DC. Of these, 31 patients developed expansion of existing hemorrhagic lesions, 13 had new contusions, three developed new extradural hemorrhages, two developed new subdural hematomas, and one patient developed an intraventricular hemorrhage. Those who developed expansion of existing hemorrhagic lesions following DC had longer ICU stays and poorer outcomes (Glasgow outcome scale).
Conclusion: After 74 DC performed in TBI patients, 67% developed new hemorrhagic lesions or expansion of previously existing hemorrhages. This finding negatively impacted clinical outcomes, including mortality.
Keywords: Decompressive craniectomy, Glasgow coma scale, Revised trauma score, Traumatic brain injury
INTRODUCTION
Decompressive craniectomy (DC) is increasingly utilized in traumatic brain injury (TBI) patients to control raised ICP refractory to best medical management.[
Here, we report a single-center experience with DC in patients with TBI by estimating the frequency, type, and clinical outcomes of hemorrhagic complications observed following the performance of DC.
MATERIALS AND METHODS
This was a retrospective review and analysis of 74 patients undergoing DC for raised ICP secondary to severe TBI from 2015 to 2017 at our tertiary care referral hospital [
Definitions and scales
We utilized the Rotterdam score to describe and standardize CT studies in these 74 patients following DC.[
Analysis
Pearson’s correlation was used to test for a correlation between RCTS at presentation and postresuscitation GCS, length of stay, and GOS. Data were analyzed using SPSS version 20 (IBM Inc, Armonk, NY, USA).
RESULTS
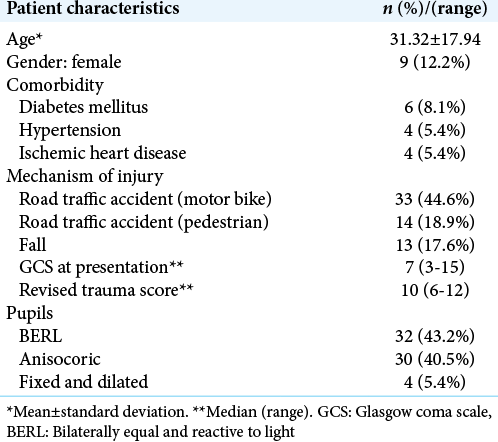
Patients demographics and clinical characteristics
A total of 74 patients undergoing DC were included in the study [
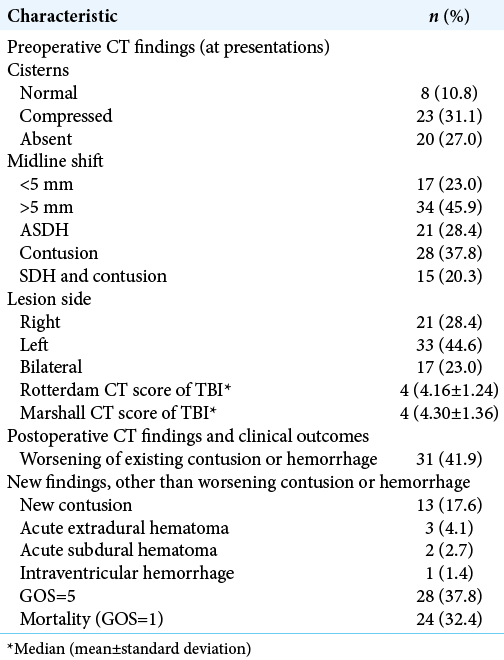
Preoperative studies showed contusions in 37.8% (n = 28) of patients, subdural hematomas in 28.4% (n = 21) of patients, while 20.3% (n = 15) of patients had both [
Surgical procedures
Surgery included in 21.6% (n = 16) bifrontal DC, in 41.9% (n = 31) unilateral DC, and in 36.5% (n = 27) DC with clot evacuation [
Postoperative new CT findings in 74 TBI patients
The postoperative CT scans performed during the index hospitalization revealed following new postoperative findings not documented on preoperative CT scans; 41.9% (n = 31) showed expansion of the previously existing contusion/ hemorrhages, 17.6% (n = 13) showed contusions that were not present on the preoperative scans, 4.1% (n = 3) developed new extradural hematomas, 2.7% (n = 2) exhibited new acute subdural hemorrhages, and 1.3% (n = 1) showed a new-onset intraventricular hemorrhage [
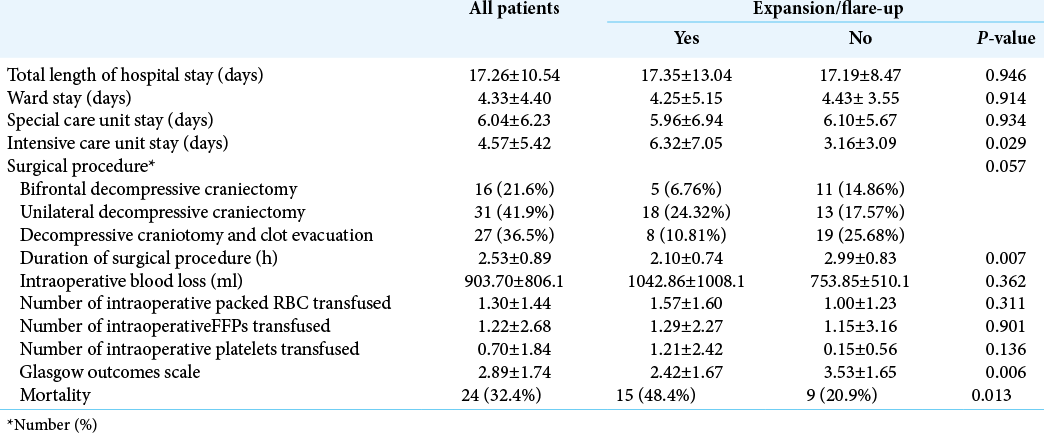
Length of hospital stay (LOS)
The overall mean floor and special care unit stay were 4.33 days (±4.40) for the no expansion/flare-up group versus 6.04 days (±6.23) for those who developed expansion/ flare of the original TBI; there was no significant difference between these two groups [
Patient outcomes
Out of the cohort of 74 patients, 37.8% (n = 28) had GOS of 5 with a mortality rate of 32.4% (n = 24) over the mean follow-up duration of 10.88 (±14.88) months [
DISCUSSION
Incidence of CT-documented contusion expansion following TBI
In this study, the decision for DC decompression was based on the CT scan findings and the patients’ clinical status; 50% of patients in groups RCTS 5 or 6 developed worsening of their primary lesions. Flint et al. reported contusion expansion in 80% of their patients with initial RCTS of 5–6.[
LOS
In the literature, the LOS for moderate-to-severe TBI patients ranged from 5.9 to 17.5 days and is proportional to the severity of the injuries.[
CT-documented complications of DC
The literature documents multiple complications of DC; primary contusion expansion, the formation of subdural hematoma subdural hygroma, and plus others.[
Most RCTS on DC remain inconclusive in terms of meaningful neurological and functional benefits of DC in patients with severe TBI. One repeated argument against DC is that the expansion of the brain causes damage to white matter tracts and allows the contusions to expand, the latter shown in our series in two-thirds of cases. Although these patients had poorer outcomes in our study, our methodology has limitations (retrospective data collection, lack of functional outcomes, lack of randomization, etc.), and it is, therefore, beyond the scope of this paper to discuss whether our findings can be extrapolated to comment on the benefits or lack thereof, of DC for hemorrhagic contusions.
CONCLUSION
In our series of 74 TBI patients, the expansion of hemorrhagic contusions was seen in 67% of patients after DC. Patients who had no expansion were more likely to have undergone clot evacuation along with DC, which were more likely to have had longer operative time, shorter length of ICU stay, and overall better outcomes including mortality.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Flint AC, Manley GT, Gean AD, Hemphill JC, Rosenthal G. Post-operative expansion of hemorrhagic contusions after unilateral decompressive hemicraniectomy in severe traumatic brain injury. J Neurotrauma. 2008. 25: 503-12
2. Kurland DB, Khaladj-Ghom A, Stokum JA, Carusillo B, Karimy JK, Gerzanich V. Complications associated with decompressive craniectomy: A systematic review. Neurocrit Care. 2015. 23: 292-304
3. Lagbas C, Bazargan-Hejazi S, Shaheen M, Kermah D, Pan D. Traumatic brain injury related hospitalization and mortality in California. Biomed Res Int. 2013. 2013: 143092
4. McGarry LJ, Thompson D, Millham FH, Cowell L, Snyder PJ, Lenderking WR. Outcomes and costs of acute treatment of traumatic brain injury. J Trauma. 2002. 53: 1152-9
5. Myburgh JA, Cooper DJ, Finfer SR, Venkatesh B, Jones D, Higgins A. Epidemiology and 12-month outcomes from traumatic brain injury in Australia and New Zealand. J Trauma. 2008. 64: 854-62
6. Stiver SI. Complications of decompressive craniectomy for traumatic brain injury. Neurosurg Focus. 2009. 26: E7
7. Waqas M, Shamim MS, Enam SF, Qadeer M, Bakhshi SK, Patoli I. Predicting outcomes of decompressive craniectomy: Use of rotterdam computed tomography classification and marshall classification. Br J Neurosurg. 2016. 30: 258-63