- Department of Neurosurgery, University of South Dakota Sanford School of Medicine, Sioux Falls, South Dakota, United States.
- Department of Pathology, University of South Dakota Sanford School of Medicine, Sioux Falls, South Dakota, United States.
Correspondence Address:
Dallas J. Soyland
Department of Neurosurgery, University of South Dakota Sanford School of Medicine, Sioux Falls, South Dakota, United States.
DOI:10.25259/SNI_786_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Dallas J. Soyland1, Dylan R. Goehner1, Kayla M. Hoerschgen2, Troy D. Gust1, Shawn M. Vuong1. Hemorrhagic spinal melanotic schwannoma presenting as acute chest pain: A case report and literature review. 14-Apr-2021;12:164
How to cite this URL: Dallas J. Soyland1, Dylan R. Goehner1, Kayla M. Hoerschgen2, Troy D. Gust1, Shawn M. Vuong1. Hemorrhagic spinal melanotic schwannoma presenting as acute chest pain: A case report and literature review. 14-Apr-2021;12:164. Available from: https://surgicalneurologyint.com/surgicalint-articles/10709/
Abstract
Background: Melanotic schwannoma (MS) is a rare variant of peripheral nerve sheath tumor. MS commonly arises along the spinal nerve sheath. Patients most often experience pain along the dermatome of the affected nerve root. Symptoms development is usually insidious. About half of MS cases are associated with Carney complex, a multi-neoplastic disorder. The remaining cases arise spontaneously. About 10–44% of these tumors undergo malignant transformation.
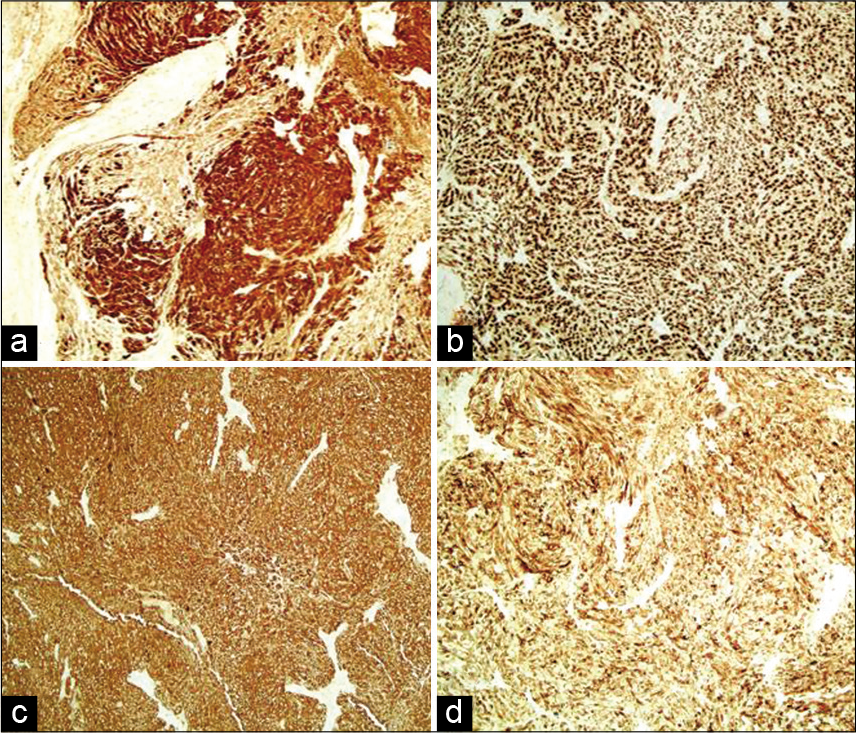
Case Description: We describe a case of hemorrhagic MS presenting as acute chest pain mimicking myocardial infarction, a presentation which has not yet been described in the literature. Neurologic examination did not reveal any abnormalities. Myocardial infarction was ruled out in the ER, and a chest CT angiogram was ordered for evaluation of PE or aortic dissection which revealed an intradural extramedullary dumbbell-shaped mass extending through the left vertebral foramen at the level of T8. MRI revealed a heterogenous mass that was hyperintense with T2 and hypointense with T1-weighted imaging. The patient underwent an open laminectomy of the left T8 and T9 vertebrae and gross total resection (GTR) of a hemorrhagic black tumor. Microscopic examination showed fascicles and nests of plump spindle cells with variable intracellular melanin. Immunohistochemistry showed the cells to be positive for S100, SOX10, HMB-45, and MART-1, confirming diagnosis of MS. Two months after the operation, the patient was doing well and is free of recurrence.
Conclusion: GTR is considered the optimal treatment for MS; radiotherapy and chemotherapy may be considered but have not been shown to improve patient outcomes.
Keywords: Carney complex, Melanotic schwannoma, Nerve sheath, Spinal neoplasia
INTRODUCTION
Melanotic schwannoma (MS) is a rare neoplastic lesion, comprising less than 1% of all nerve sheath tumors.[
Because of the scarcity of MS cases reported in the literature, demographic and clinical data on this entity are continuing to evolve. Prior reviews have reported a male-female ratio of 1.1:1 with the highest frequency in the fourth decade.[
The presentation of MS is variable and dependent on the location of the tumor and involvement of local structures. As most spinal schwannomas arise from the spinal nerve sheath,[
Half of MS cases are related to the Carney complex, an autosomal dominant inheritance multi-neoplastic syndrome resulting from a PRKAR1A gene mutation.[
Historically, Carney complex-associated and sporadic MS have been reviewed together with data analysis including both etiologies.[
Here, we discuss a case of sporadic hemorrhagic spinal MS with the only known presentation of sudden onset chest pain mimicking myocardial infarction as well as a literature review of all reported cases of sporadic spinal MS in an attempt to expand on previously reported demographic and clinical data, as well as proper diagnosis and treatment.
CASE PRESENTATION
A 53-year-old man reported to the emergency room with a 2-day history of sudden-onset left chest pain radiating to his left back. The pain was intermittent over the 48 h and felt similar to the pain he experienced in a prior episode of pleurisy. It did not localize to any specific region of the chest and did not involve the left arm. It was partially relieved with NSAID use and resting on his left side. The patient had a 30 pack year smoking history as well as a history of illicit drug use. He had history of hypertension that was controlled with lifestyle changes and did not show any signs of end organ damage. The previous clinic visits showed his blood pressure to be under control and on admission it was 100/78. A review of systems and physical examination did not show any signs of paresthesias, numbness, weakness, or ataxia. Cardiovascular, respiratory, and neurologic physical exams were normal. The patient was given nitroglycerin and fentanyl, which eased the pain. Because of both his history of smoking and hypertension as well as his clinical presentation, a chest X-ray, EKG, and blood work-up including troponin I and D-dimer were performed which returned normal results.
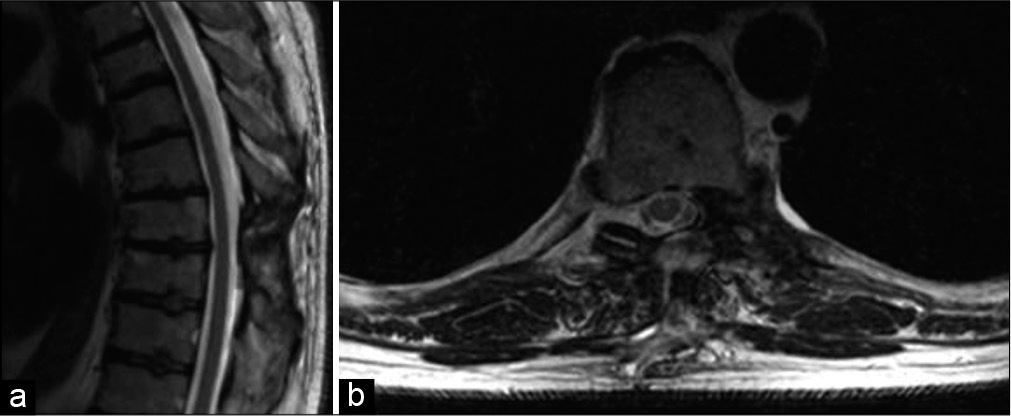
Due to high suspicion for coronary ischemia and other cardiac etiologies related to his history of smoking, hypertension, and illicit drug use, the patient was admitted to further investigate his chest pain. At this point, his pain was completely resolved with fentanyl. As part of expanding the differential diagnosis for chest pain, to rule out pulmonary embolism and aortic dissection, a CT angiogram of the chest was performed and revealed a 4.4 × 2 × 2.1 cm soft-tissue mass compressing the spinal cord at the level of T8-T9. To further characterize the spinal lesion, MRI imaging was obtained and confirmed the presence of a heterogeneous mass at the left T8 that was hyperintense on T2-weighted and hypointense on T1-weighted images [
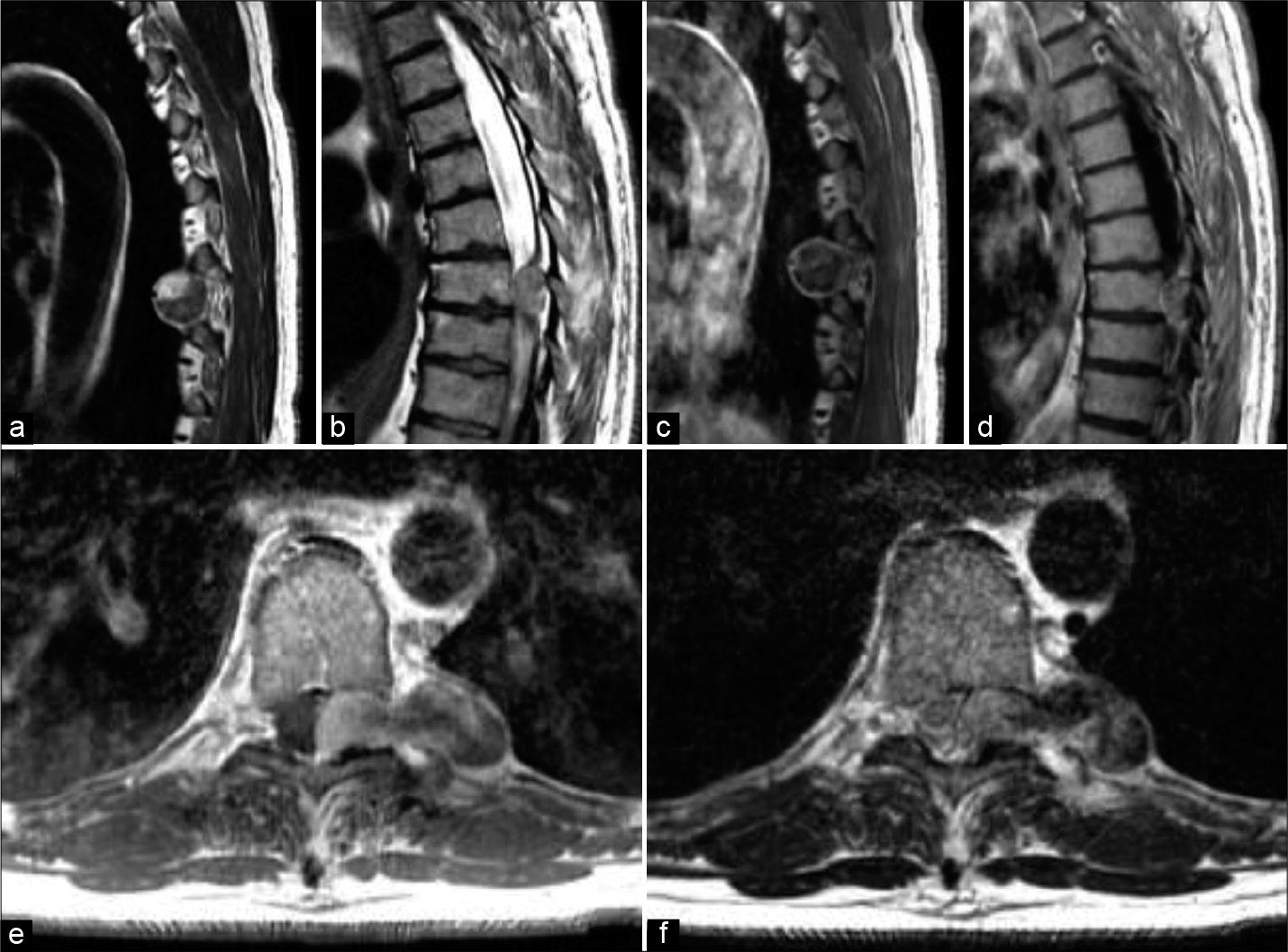
Figure 1:
(a and b) Sagittal T2-weighted MRI scans taken at the time of presentation show a slightly hyperintense heterogeneous mass at the level of T8-T9. (c and d) T1-weighted MRI scans taken after gadolinium administration show a hypointense mass with circumferential enhancement. Axial T1 (e) and T2 (f) images show an extramedullary intradural dumbbell-shaped mass.
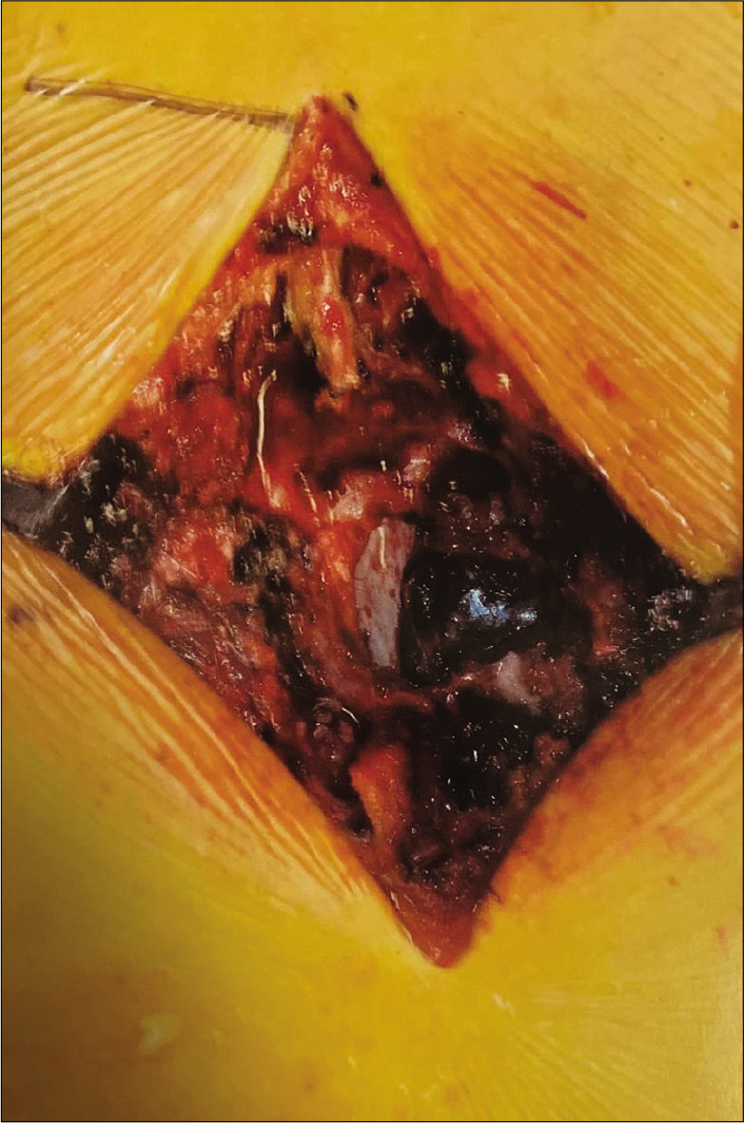
Given the significant mass effect of the mid-thoracic spinal cord and pain symptoms, the patient underwent an open laminectomy and partial facetectomy of T8 and T9. A dark, dumbbell-shaped mass could be seen extending from the left spinal column grossly [
Figure 3:
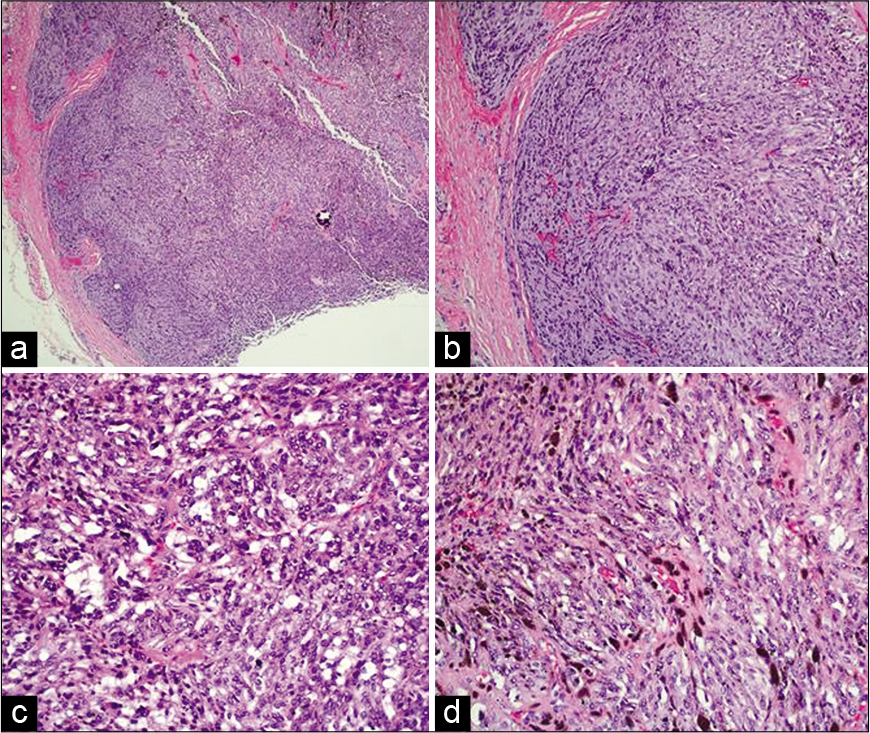
(a) Tumor is circumscribed with cells in fascicles and nests. (b) Fascicles and nests composed of plump spindle cells. (c) Adipose-like cells admixed with tumor cells. (d) Melanin pigment deposition in the tumor cells (hematoxylin-eosin, original magnifications ×40 (a), ×100 (b), ×200 (c and d)).
A complete history and review of systems did not reveal any family history or clinical signs of Carney complex in this patient. He was discharged 1 day after surgery. We followed up with the patient 2 months after surgery. He was doing well other than some persisting incisional pain. Imaging did not show any local recurrence of the tumor [
DISCUSSION
Sporadic spinal MSs are a rare entity. As more cases are reported, demographic and clinical data are evolving and may change evaluation and treatment of MS. Some prior case reports of MS do not provide in-depth data on patient symptoms, treatment, and follow-up.[
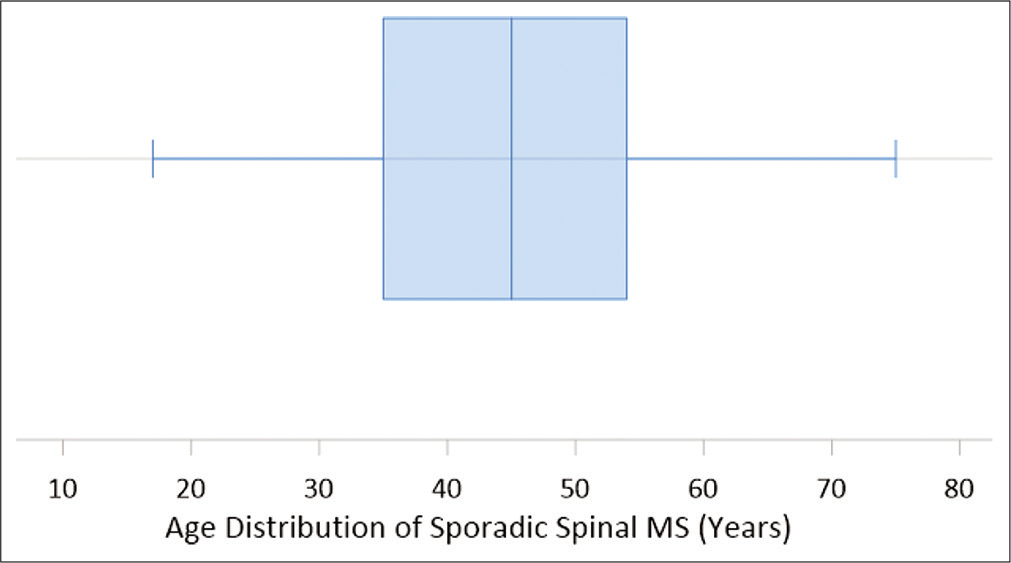
MS is considered a tumor of young adults, with a reported mean age of 38 years.[
MS can occur anywhere along the peripheral nerve sheaths.[
Although several theories have been proposed, the etiology of MS is unknown. Some of the more popular theories include melanomatous transformation of neoplastic Schwann cells, phagocytosis of melanin by Schwann cells, two distinct proliferating cell lines of Schwann cells and melanocytes, and a genetic mutation to a common precursor of melanocytes and Schwann cells as they both arise from neuroectoderm and the cells migrate together.[
Half of MS cases are related to the Carney complex, an autosomal dominant inheritance multi-neoplastic syndrome resulting from a PRKAR1A gene mutation.[
The presenting symptoms of MS are highly dependent on the location of the tumor. Spinal MS most commonly presents with pain correlating with the affected dermatome, often accompanied by paresthesias and muscle weakness of the same region.[
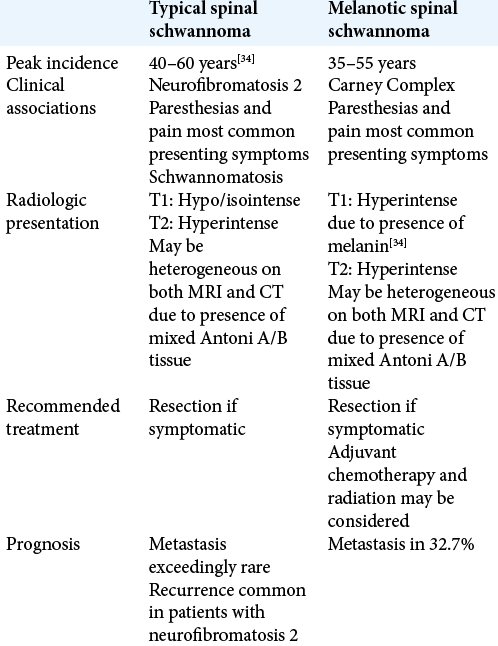
Common radiologic features of MS include a hyperintense signal on T1-weighted MRI and a variable isointense to hyperintense signal on T2-weighted images.[
On gross examination, the tumors have been described as dark brown or black in color, sometimes with hemorrhagic components, cyst formation, or necrosis.[
Classical morphology of MS includes sheets of spindled and epithelioid cells with fascicles of eosinophilic cytoplasm, occasional psammoma bodies, and melanosomes in various stages of maturation within neoplastic cells.[
At this moment, the only clinical or pathological factors predictive for the prognosis of MS are mitotic activity[
GTR with or without radiation therapy is the favored treatment for MS.[
CONCLUSION
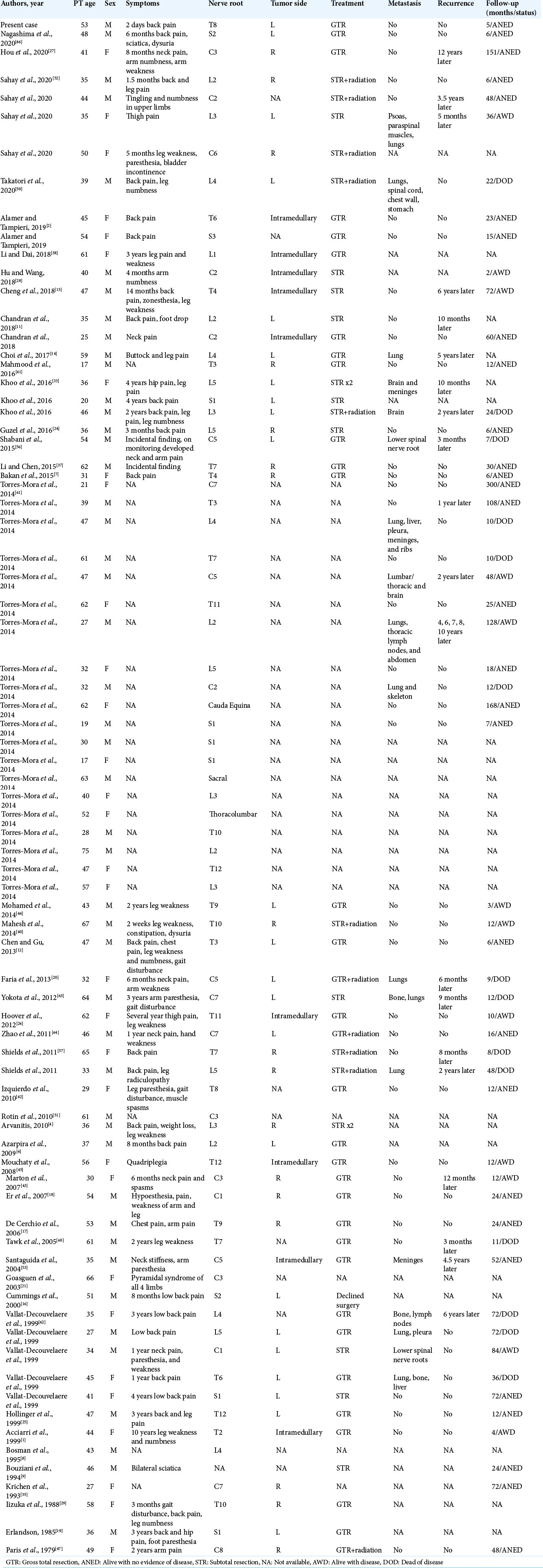
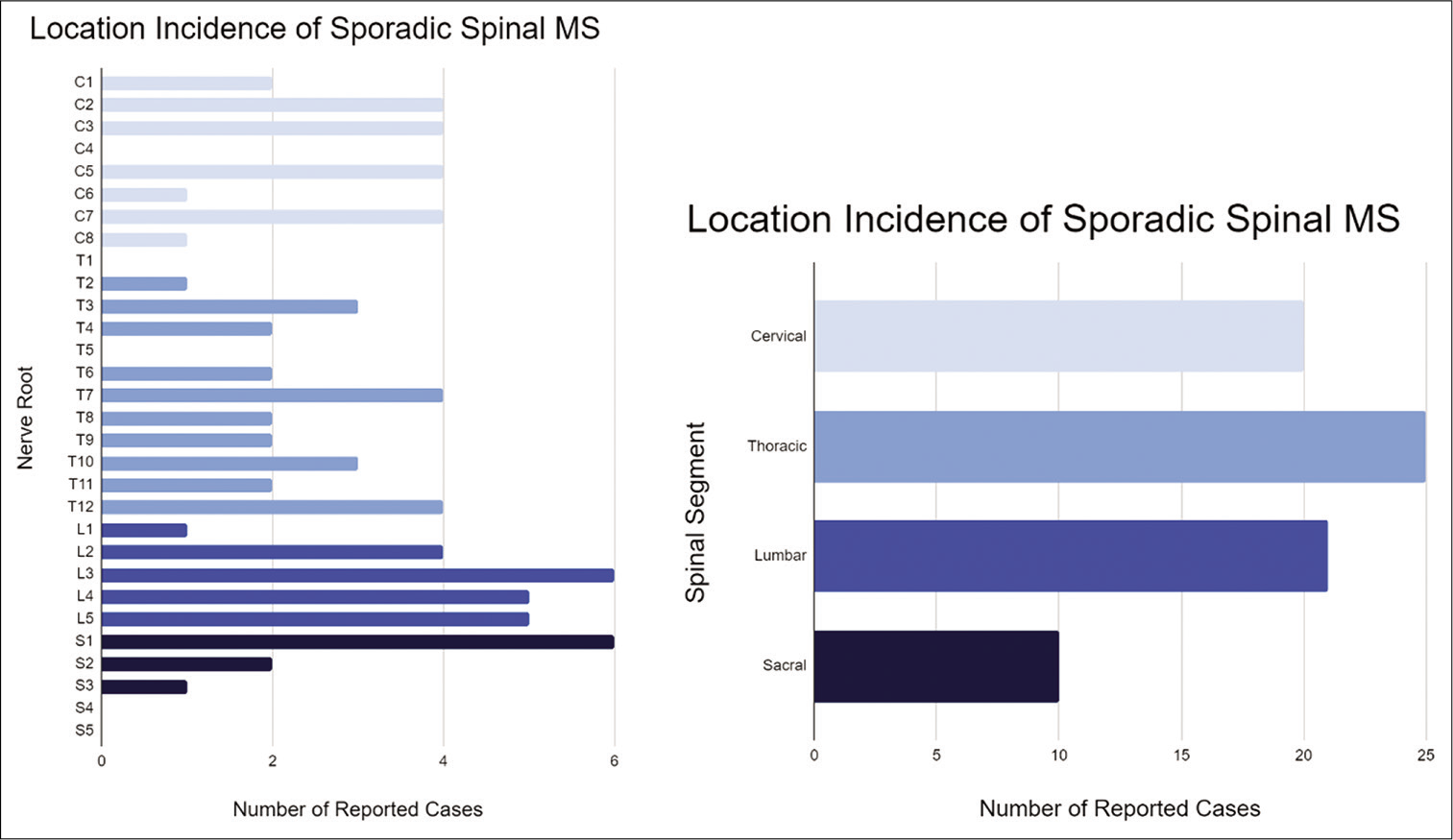
MS is a rare neoplasm that is often associated with Carney complex but develops sporadically in about half of reported cases. Seventy-nine cases of sporadic MS arising along the spine have now been described. The presentation of spinal MS varies but most commonly includes an insidious onset of back, leg, or neck pain associated with the affected dermatome over months to years. Our case represents the only case of MS to date that presented as acute chest pain mimicking myocardial infarction and suggests that hemorrhagic spinal lesions should be considered in the differential diagnosis of acute chest pain, especially when cardiac workup is negative. Our review of sporadic MS cases showed a male preference as well as an average age of 44 years, slightly older than previously described. We also found that 11% of reported cases of sporadic spinal MS were intramedullary. Immunohistochemical staining should be used to differentiate MS from malignant melanoma. Gross total excision with long-term serial imaging is recommended.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Acciarri N, Padovani R, Riccioni L. Intramedullary melanotic schwannoma. Report of a case and review of the literature. Br J Neurosurg. 1999. 13: 322-5
2. Alamer A, Tampieri D. Brain and spine melanotic schwannoma: A rare occurrence and diagnostic dilemma. Neuroradiol J. 2019. 32: 335-43
3. Alexiev BA, Chou PM, Jennings LJ. Pathology of melanotic schwannoma. Arch Pathol Lab Med. 2018. 142: 1517-23
4. Arvanitis LD. Melanotic schwannoma: A case with strong CD34 expression, with histogenetic implications. Pathol Res Pract. 2010. 206: 716-9
5. Asazuma T, Toyama Y, Maruiwa H, Fujimura Y, Hirabayashi K. Surgical strategy for cervical dumbbell tumors based on a three-dimensional classification. Spine (Phila Pa 1976). 2004. 29: E10-4
6. Azarpira N, Torabineghad S, Sepidbakht S, Rakei M, Bagheri MH. Cytologic findings in pigmented melanotic schwannoma: A case report. Acta Cytol. 2009. 53: 113-5
7. Bakan S, Kayadibi Y, Ersen E, Vatankulu B, Ustundag N, Hasiloglu ZI. Primary Psammomatous Melanotic Schwannoma of the Spine. Ann Thorac Surg. 2015. 99: e141-3
8. Bosman C, Boldrini R, Corsi A. Malignant melanotic schwannoma or schwannian melanoma?. Tumori. 1995. 81: 208-12
9. Bouziani A, Kammoun N, Zidi B, Ben Hamadi F, Benzarti H, Khelil A. Melanotic schwannoma. A case with review of the literature. Arch Anat Cytol Pathol. 1994. 42: 46-53
10. Carney JA. Psammomatous melanotic schwannoma. A distinctive, heritable tumor with special associations including cardiac myxoma and the Cushing syndrome. Am J Surg Pathol. 1990. 14: 206-22
11. Chandran RS, Patil AK, Prabhakar RB, Balachandran K. Melanotic schwannoma of spine: Illustration of two cases with diverse clinical presentation and outcome. Asian J Neurosurg. 2018. 13: 881-4
12. Chen D, Gu W. Subdural extramedullary melanotic schwannoma of the thoracic spinal cord: A case report. Turk Neurosurg. 2015. 25: 326-31
13. Cheng X, Liu J, Le J, Huang S, Chen H, You C. Invasive intramedullary melanotic schwannoma: Case report and review of the literature. Eur Spine J. 2018. 27: 303-8
14. Choi SE, Cha YJ, Kim J, Cha H, Seo J, Kuh SU. A rare case of aggressive melanotic schwannoma occurred in spinal nerve of a 59-year-old male. J Pathol Transl Med. 2017. 51: 505-8
15. Cohen JN, Yeh I, LeBoit PE. Melanotic schwannoma of the vulva: A case report and review of the literature. Am J Dermatopathol. 2020. 42: 46-51
16. Cummings TJ, Liu K, Jordan LK, Dodd LG. Fine-needle aspiration diagnosis of psammomatous melanotic schwannoma. Diagn Cytopathol. 2000. 23: 55-8
17. De Cerchio L, Contratti F, Fraioli MF. Dorsal dumb-bell melanotic schwannoma operated on by posterior and anterior approach: Case report and a review of the literature. Eur Spine J. 2006. 15: 664-9
18. Er U, Kazanci A, Eyriparmak T, Yigitkanli K, Senveli E. Melanotic schwannoma. J Clin Neurosci. 2007. 14: 676-8
19. Erlandson RA. Melanotic schwannoma of spinal nerve origin. Ultrastruct Pathol. 1985. 9: 123-9
20. Faria MH, Doria-Netto RH, Osugue GJ, Lde SQ, ChaddadNeto FE. Melanotic schwannoma of the cervical spine progressing with pulmonary metastasis: Case report. Neurol Med Chir (Tokyo). 2013. 53: 712-6
21. Goasguen O, Boucher E, Pouit B, Soulard R, Le Charpentier M, Pernot P. Melanotic schwannoma, a tumor with a unpredictable prognosis: case report and review of the literature. Neurochirurgie. 2003. 49: 31-8
22. Greenberg M.editors. Handbook of Neurosurgery. Thieme Publishers: New York; 2020. p. 187
23. Gulati HK, Joshi AR, Anand M, Deshmukh SD. Non psammomatous melanocytic schwannoma presenting as a subcutaneous nodule: A rare presentation of a rare lesion. Asian J Neurosurg. 2016. 11: 317-8
24. Guzel E, Er U, Guzel A, Toktas Z, Yapicier O. Melanotic schwannoma of the L5 root. Neuroradiol J. 2016. 29: 219-21
25. Hollinger P, Godoy N, Sturzenegger M. Magnetic resonance imaging findings in isolated spinal psammomatous melanotic schwannoma. J Neurol. 1999. 246: 1100-2
26. Hoover JM, Bledsoe JM, Giannini C, Krauss WE. Intramedullary melanotic schwannoma. Rare Tumors. 2012. 4: e3
27. Hou Z, Shi T, Li G, Tian L, Li X, Liu X. Extramedullary melanotic schwannoma recurrence in the cervical vertebral arch: A case report and review of the literature. J Int Med Res. 2020. 48: 300060520947919
28. Hu L, Wang C. Intramedullary melanotic schwannoma of the cervical spine: A case report and literature review. Mol Clin Oncol. 2018. 8: 567-70
29. Iizuka H, Nakamura T, Kadoya S. Spinal melanotic schwannoma: Report of a case. No Shinkei Geka. 1988. 16: 1199-205
30. Italiano A, Michalak S, Soulie P, Peyron AC, Pedeutour F. Trisomy 6p and ring chromosome 11 in a melanotic schwannoma suggest relation to malignant melanoma rather than conventional schwannoma. Acta Neuropathol. 2011. 121: 669-70
31. Kamilaris CD, Faucz FR, Voutetakis A, Stratakis CA. Carney complex. Exp Clin Endocrinol Diabetes. 2019. 127: 156-64
32. Keskin E, Ekmekci S, Oztekin O, Diniz G. Melanotic schwannomas are rarely seen pigmented tumors with unpredictable prognosis and challenging diagnosis. Case Rep Pathol. 2017. 2017: 1807879
33. Khoo M, Pressney I, Hargunani R, Tirabosco R. Melanotic schwannoma: An 11-year case series. Skeletal Radiol. 2016. 45: 29-34
34. Koeller KK, Shih RY. Intradural extramedullary spinal neoplasms: Radiologic-pathologic correlation. Radiographics. 2019. 39: 468-90
35. Krichen H, Daghfous MS, Mrabet A, Douik M, Slimane N, Forest M. Extended melanocytic tumor of the cervical spine. Apropos of a case of melanotic schwannoma. Ann Pathol. 1993. 13: 184-7
36. Kusters-Vandevelde HV, van Engen-van Grunsven IA, Kusters B, van Dijk MR, Groenen PJ, Wesseling P. Improved discrimination of melanotic schwannoma from melanocytic lesions by combined morphological and GNAQ mutational analysis. Acta Neuropathol. 2010. 120: 755-64
37. Li B, Chen Q. Melanotic schwannoma of thoracic spinal root mimics metastatic melanoma: A potential pitfall for misdiagnosis. Int J Clin Exp Pathol. 2015. 8: 8639-41
38. Li XL, Dai SD. Melanotic schwannoma: Two cases of rare lesions. Pathol Oncol Res. 2019. 25: 1667-70
39. Mahato D, Vivas-Buitrago T, Gassie K, Jentoft M, Tavanaiepour D, Quinones-Hinojosa A. Intracranial melanotic schwannomas: A rare variant with unusual adherent features. J Neurooncol. 2018. 136: 299-306
40. Mahesh I, Karthikeyan VS, Malathi M. Spotty skin pigmentation and multiple blue naevi as cutaneous markers for spinal melanotic schwannoma. BMJ Case Rep. 2014. 2014: bcr2013201567
41. Mahmood UB, Khan FW, Fatima B, Tariq MU, Fatimi SH. Primary melanotic schwannoma with typical histology. J Coll Physicians Surg Pak. 2016. 26: 707-9
42. Izquierdo MA, Lopez-Soto V, Saenz-Santamaria J, LacruzPelea C. Intraoperative cytological findings in two cases of psammomatous melanotic schwannoma. Cytopathology. 2011. 22: 60-62
43. Marton E, Feletti A, Orvieto E, Longatti P. Dumbbell-shaped C-2 psammomatous melanotic malignant schwannoma. Case report and review of the literature. J Neurosurg Spine. 2007. 6: 591-9
44. Mohamed M, Panos S, Baborie A, Das K, Pillay R. Atypical benign melanotic thoracic intradural schwannoma. Br J Neurosurg. 2014. 28: 411-3
45. Mouchaty H, Conti R, Buccoliero AM, Conti P. Intramedullary melanotic schwannoma of the conus medullaris: A case report. Spinal Cord. 2008. 46: 703-6
46. Nagashima Y, Nishimura Y, Eguchi K, Awaya T, Yoshikawa S, Haimoto S. Intraosseous melanotic schwannoma in the sacrum mimicking primary bone tumor. NMC Case Rep J. 2020. 7: 107-111
47. Paris F, Cabanes J, Munoz C, Tamarit L. Melanotic spinothoracic schwannoma. Thorax. 1979. 34: 243-6
48. Peltier J, Page C, Toussaint P, Bruniau A, Desenclos C, Le Gars D. Melanocytic schwannomas. Report of three cases. Neurochirurgie. 2005. 51: 183-9
49. Reddy VU, Suneetha P, Shanthi V, Mohan KV, Agrawal A. Intracranial hemorrhagic metastases as the first manifestation of an occult melanoma. South Asian J Cancer. 2015. 4: 101-2
50. Rodriguez FJ, Folpe AL, Giannini C, Perry A. Pathology of peripheral nerve sheath tumors: Diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012. 123: 295-319
51. Rotin DL, Shishkina LV, Shevelev IN, Zelenkov PV. Melanotic schwannoma of C(III) spinal root. Zh Vopr Neirokhir Im N N Burdenko. 2010. 2: 33-36
52. Sahay A, Epari S, Gupta P, Goda J, Shetty P, Patil V. Melanotic schwannoma, a deceptive misnomer for a tumor with relative aggressive behavior: A series of 7 cranial and spinal cases. Int J Surg Pathol. 2020. 2020: 1066896920923146
53. Santaguida C, Sabbagh AJ, Guiot MC, Del Maestro RF. Aggressive intramedullary melanotic schwannoma: Case report. Neurosurgery. 2004. 55: 1430
54. Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008. 9: 557-68
55. Seppala MT, Haltia MJ, Sankila RJ, Jaaskelainen JE, Heiskanen O. Long-term outcome after removal of spinal schwannoma: A clinicopathological study of 187 cases. J Neurosurg. 1995. 83: 621-6
56. Shabani S, Fiore SM, Seidman R, Davis RP. Intraspinal psammomatous melanotic schwannoma not associated with carney complex: Case report. J Neurosurg Spine. 2015. 23: 233-8
57. Shields LB, Glassman SD, Raque GH, Shields CB. Malignant psammomatous melanotic schwannoma of the spine: A component of Carney complex. Surg Neurol Int. 2011. 2: 136
58. Smith AB, Rushing EJ, Smirniotopoulos JG. Pigmented lesions of the central nervous system: Radiologic-pathologic correlation. Radiographics. 2009. 29: 1503-24
59. Takatori N, Hiyama A, Sakai D, Katoh H, Sato M, Watanabe M. A rare case of intraspinal psammomatous melanotic schwannoma: A case report. Spine Surg Relat Res. 2020. 4: 91-4
60. Tawk RG, Tan D, Mechtler L, Fenstermaker RA. Melanotic schwannoma with drop metastases to the caudal spine and high expression of CD117 (c-kit). J Neurooncol. 2005. 71: 151-6
61. Torres-Mora J, Dry S, Li X, Binder S, Amin M, Folpe AL. Malignant melanotic schwannian tumor: A clinicopathologic immunohistochemical, and gene expression profiling study of 40 cases, with a proposal for the reclassification of “melanotic schwannoma”. Am J Surg Pathol. 2014. 38: 94-105
62. Vallat-Decouvelaere AV, Wassef M, Lot G, Catala M, Moussalam M, Caruel N. Spinal melanotic schwannoma: A tumour with poor prognosis. Histopathology. 1999. 35: 558-66
63. Yokota H, Isobe K, Murakami M, Kubosawa H, Uno T. Dumbbell-shaped nonpsammomatous malignant melanotic schwannoma of the cervical spinal root. Spine J. 2012. 12: e14-17
64. Zhao QH, Zhi S, Wang Z, Tian JW. Psammomatous melanotic schwannoma with cystic changes from old hemorrhages in the cervical spinal canal: A case report. Orthop Surg. 2011. 3: 143-6