- Heaven-Gemini International Collaborative Group, Heaven Gemini Global Initiative, Turin, Italy.
DOI:10.25259/SNI_395_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Canavero S, Ren X, Kim C. Heterologous spinal cord transplantation in man. Surg Neurol Int 21-Jun-2021;12:295
How to cite this URL: Canavero S, Ren X, Kim C. Heterologous spinal cord transplantation in man. Surg Neurol Int 21-Jun-2021;12:295. Available from: https://surgicalneurologyint.com/surgicalint-articles/10902/
Despite a slew of press releases from academic research groups (and naive media) over the past 40 years (starting with the Aguayo studies in the early 1980s) announcing the imminent arrival of a cure for spinal paralysis (paraplegia and tetraplegia), the failure of contemporary spinal cord injury (SCI) research stands out egregiously to the eyes of the many patients confined to their wheelchairs. Pace stem cells and a smorgasbord of other “successful” (in rodents) treatments reported in “prestigious” academic journals (e.g., Nature, Science, and many others).[1] It is not for us to answer the question of what went so awfully wrong (certainly not a lack of funds or support), but it behooves us to bring to everyone’s attention the truly promising avenue that must be trodden to achieve the long-sought cure. Over the past 70 years, clinical transplantation has made it clear that whenever a body part cannot be fixed by available medical treatments, this body part can be replaced, be it an organ or an appendage. A rather simple, but powerful, assumption. SCI is generally due to localized tissue disruption. Lost function might thus be recovered by replacing the injured segment of the spinal cord [Figure 1]. To achieve this goal, four obstacles must be cleared:
- Sourcing a homotopic segment of healthy spinal cord
- Development of a technology that allows anatomophysiologic integration of the cord transplant (both the cord proper and its roots) with the undamaged cord of the patient
- Availability of a microrevascularization technology
- Immunologic rejection.
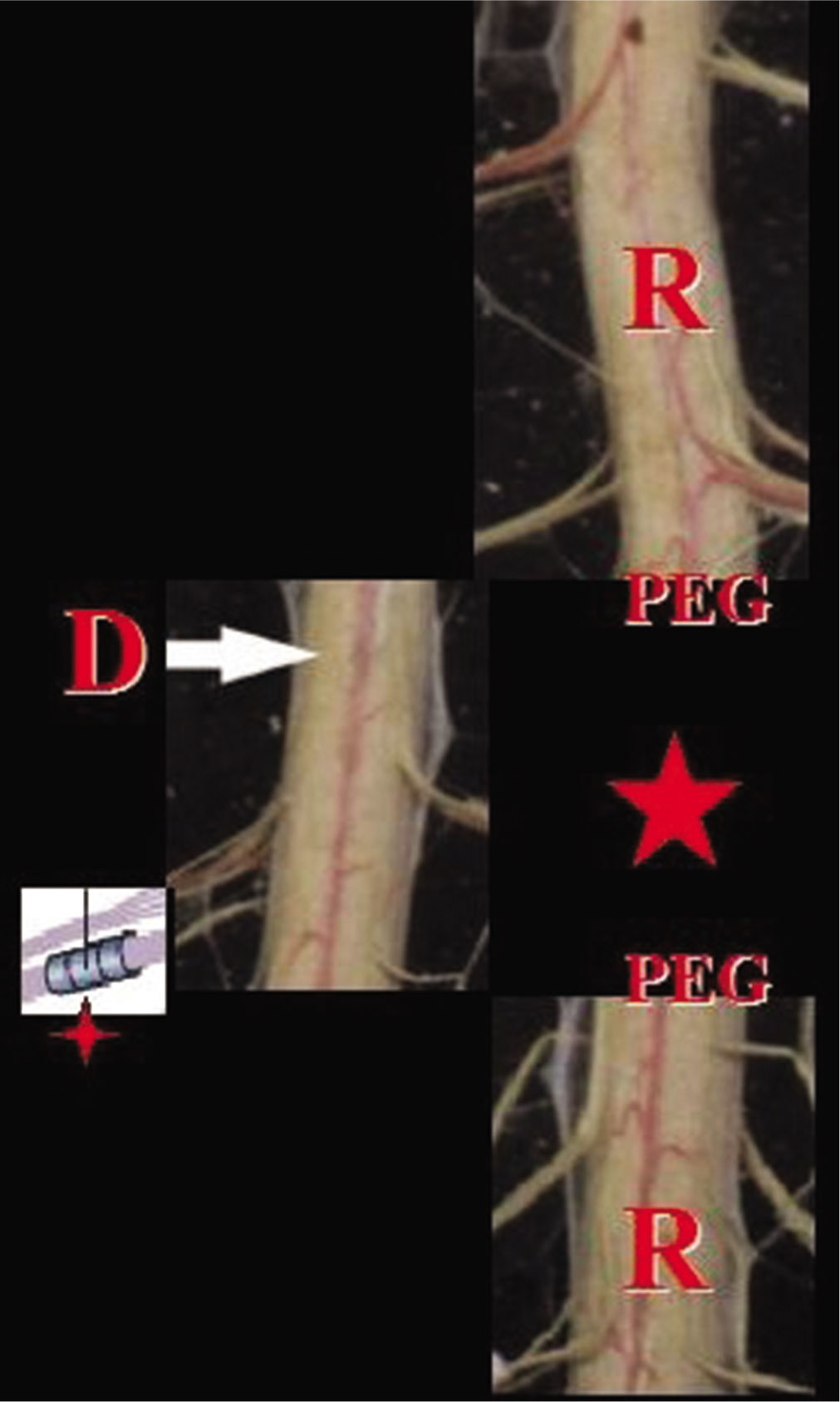
Figure 1:
Recipient (r)’s damaged cord segment is removed (star) and a healthy segment is inserted (arrow). Roots are anastomosed with sutureless techniques (four-point star), polyethylene glycol is applied (a spinal cord stimulation apparatus is added after dural closure) and revascularization follows.
SOURCE OF TRANSPLANTABLE CORD
The idea of replacing a damaged portion of the spinal cord with a healthy one is not new. In 1905, Shirres reported his attempt to graft a segment of healthy canine cord in a human paraplegic patient: initial sensory recuperation was observed at 3 months, but the patient succumbed to infection; of relevance, autopsy showed clear signs of neuroregeneration.[
In the XXI century, cord segments dovetailing the injured patient’s cord can be harvested from brain-dead organ donors at the same level, thereby respecting the intrinsic anatomy of the damaged cord. A special instrument (GEMIN-o-tome) would allow quick dissection.[
INTEGRATION OF THE CORD TRANSPLANT
The GEMINI spinal cord fusion protocol enables recovery of sensorimotor function after full cord transection in rodents, canines, and primates (fully reviewed in Canavero et al.[
Fusion of the cord proper would be followed by reconnection of the dorsal and ventral roots. Spinal nerve root anastomosis is not a new concept. Pioneered by Kilvington in 1907, in the 1960s, Carlsson and Sundin were the first to report on homotopic intradural reconstruction of severed sacral ventral roots using direct repair combined with a tubulization technique: functional reinnervation was demonstrated, including – by later work – in man.[
MICROREVASCULARIZATION
The transplanted segment must be revascularized. Part and parcel of the GEMINI protocol is polyethylene glycol, a polymer that has been shown to promote angiogenesis in an SCI model.[
IMMUNOREJECTION
As per other transplant procedures, immunosuppression is indicated, at least initially. Given the minimal amount of foreign tissue that is inserted into the patient, it is likely that immunosuppression would require a lighter dosing of drugs than, say, face transplants, minimizing its associated toxicity. It must be noted that there are differences between the brain and the cord in neuroimmunological terms, with greater neuroinflammatory responses in the cord.[
From this short survey, it is clear that a cure for spinal paralysis is possible. It is only hoped that the international neurosurgical community does not take another 40 years to bring it to fruition.
References
1. Canavero S, Bonicalzi V. Fall of the titans. The demise of basic neuroscience research. Engineering. 2015. 1: 409
2. Canavero S, Ren XP.editors. The Technology of Head Transplantation. New York: Nova Science Publishers; 2020. p.
3. Canavero S. Extreme Brain Reanimation. Available from: https://wwwamazon.com/extreme-brainreanimation-frankenstein-effect-ebook/dp/b08bdhht54ref=sr_1_9?dchild=1&keywords=sergio+canavero&qid=1600767047&s=books&sr=1-9.
4. Canavero S. The Technology of Brain Transplantation. Available from: https://www.amazon.com/technology-brain-transplantation-sergio-canavero/dp/b08bdwy9q2/ref=sr_1_6?keywords=sergio+canavero&qid=1599227917&sr=8-6.
5. Frey M, Happak W, Girsch W, Bittner R, Gruber H. Histomorphometric studies in patients with facial palsy treated by functional muscle transplantation: New aspects for the surgical concept. Ann Plast Surg. 1991. 26: 370-9
6. Gomez-Amaya SM, Barbe MF, de Groat WC, Brown JM, Tuite GF, Corcos J. Neural reconstruction methods of restoring bladder function. Nat Rev Urol. 2015. 12: 100-18
7. Liu Y, Zhou X, Ma J, Ge Y, Cao X. The diameters and number of nerve fibers in spinal nerve roots. J Spinal Cord Med. 2015. 38: 532-7
8. Qassemyar Q, Gianfermi M. Supermicrosurgery and hyaluronic acid: Experimental feasability study of a new method. Ann Chir Plast Esthet. 2015. 60: e59-65
9. Ren X, Kim CY, Canavero S. Bridging the gap: Spinal cord fusion as a treatment of chronic spinal cord injury. Surg Neurol Int. 2019. 10: 51
10. Ren XP, Henderson P, Kim CY, Canavero S, Preedy VR, Rajendram R, Martin S.editors. GEMINI-supported spinal cord transplantation for the treatment of chronic spinal paralysis. Overview and initial clinical translation. Diagnosis and Treatment of Spinal Cord Injury. Amsterdam: Elsevier, Academic Press; 2021. p.
11. Schalow G. Feeder arteries, longitudinal arterial trunks and arterial anastomoses of the lower human spinal cord. Zentralbl Neurochir. 1990. 51: 181-4
12. Sindou M, Chignier E, Mazoyer JF, Pialat J, Fischer G, Descotes J. Revascularization of the spinal cord by micro-anastomoses in dogs. Surg Neurol. 1979. 12: 492-5
13. Woolsey D, Minckler J, Rezende N, Klemme R. Human spinal cord transplantation. Exp Med Surg. 1944. 2: 93-102
14. Xuan FL, Chithanathan K, Lilleväli K, Yuan X, Tian L. Differences of microglia in the brain and the spinal cord. Front Cell Neurosci. 2019. 13: 504
15. Zhang B, Gensel JC. Is neuroinflammation in the injured spinal cord different than in the brain? Examining intrinsic differences between the brain and spinal cord. Exp Neurol. 2014. 258: 112-20