- Chief of Neurosurgical Spine/Education, NYU Winthrop Hospital, Mineola, New York, USA
Correspondence Address:
Nancy E. Epstein
Chief of Neurosurgical Spine/Education, NYU Winthrop Hospital, Mineola, New York, USA
DOI:10.4103/sni.sni_248_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy E. Epstein. High lumbar noninstrumented fusion rates using lamina autograft and Nanoss/bone marrow aspirate. 20-Jul-2017;8:153
How to cite this URL: Nancy E. Epstein. High lumbar noninstrumented fusion rates using lamina autograft and Nanoss/bone marrow aspirate. 20-Jul-2017;8:153. Available from: http://surgicalneurologyint.com/surgicalint-articles/high-lumbar-noninstrumented-fusion-rates-using-lamina-autograft-and-nanossbone-marrow-aspirate/
Abstract
Background:Patients with marked osteoporosis and/or obesity/morbid obesity and severe multilevel lumbar stenosis and other pathology often undergo multilevel laminectomies with non instrumented posterolateral fusions (PLF). The other pathology may include combinations of degenerative spondylolisthesis/lysis, foraminal/far lateral discs, and/or synovial cysts requiring more extensive facet resections. Presently, spine surgeons often use bone graft expanders to supplement the lamina autograft harvested in the course of laminectomy/decompressions for the PLF mass.
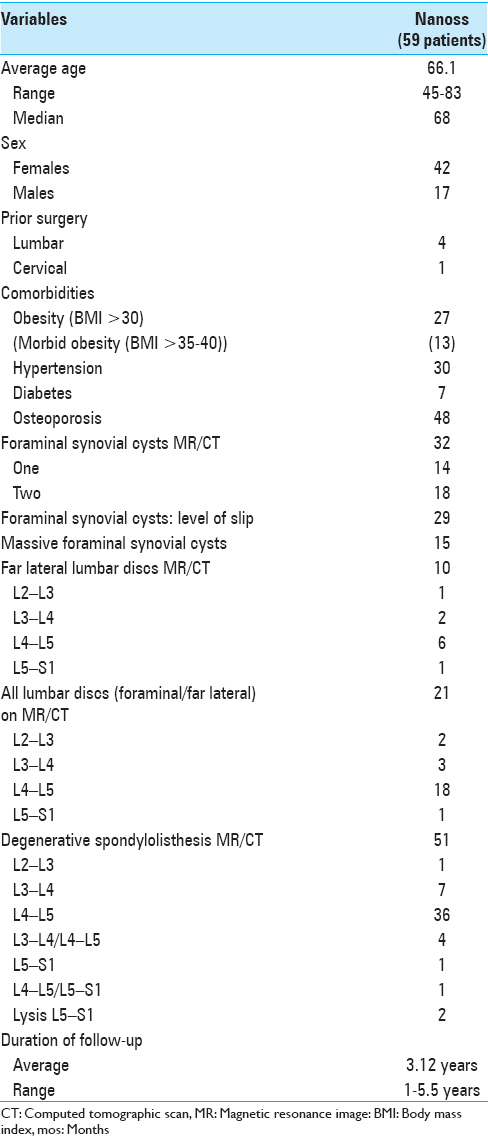
Methods:In 59 patients, we prospectively analyzed the fusion rates following multilevel laminectomies/noninstrumented fusions using lamina autograft and the bone graft expander Nanoss (RTI Surgical Alachua, FL, and USA) with autogenous bone marrow aspirate (BMA). Patients averaged 66.1 years of age; many exhibited marked osteoporosis (48 patients) and obesity (13 of 27 morbidly obese). Magnetic resonance (MR) and computed tomography (CT) studies documented stenosis/ossified yellow ligament (OYL) and degenerative spondylolisthesis (51 patients)/lysis (2 patients), synovial cysts (32 patients), and disc herniations (10 of 21 far lateral). Patients were followed remove up for an average of 3.12 years.
Results:Average 4.0 level laminectomies/1.2 level noninstrumented fusions utilized lamina autograft and Nanoss/BMA. Both X-ray/CT studies performed an average of 4.9 months postoperatively documented a 97% fusion rate (57 of 59 patients). Two patients with severe osteoporosis, morbid obesity, and smoking histories exhibited pseudarthroses; neither was sufficiently symptomatic to require secondary surgery.
Conclusions:Fifty-nine patients with multilevel lumbar stenosis/OYL and other pathology underwent multilevel lumbar laminectomies/noninstrumented fusions using lamina autograft and Nanoss/BMA. Both dynamic X-ray/CT studies confirmed a 97% fusion rate an average of 4.9 months postoperatively. Nanoss/BMA contributed to a high posterolateral lumbar non instrumented fusion rate without complciations.
Keywords: Bone marrow aspirate, non instrumented fusions, nanoss
INTRODUCTION
Following the prospective performance of 59 multilevel lumbar laminectomies for stenosis/ossification of the yellow ligament, patients underwent partial non instrumented posterolateral lumbar fusions (PLF). Notably, many patients were osteoporotic and/or obese/morbidly obese. Many PLF required additional partial/full facetectomies to address combinations of degenerative spondylolisthesis, spondylolysis, lateral/foraminal/far lateral discs, and synovial cysts and/or disc herniations [Figures
Figure 3
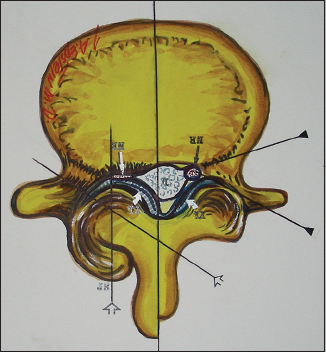
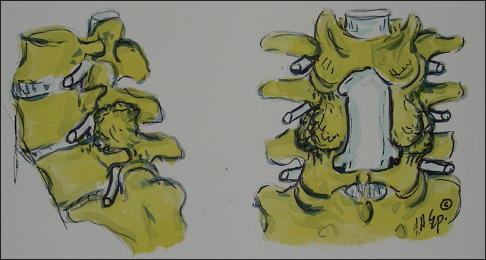
Axial illustration (Joseph A. Epstein M.D., copyright Nancy E. Epstein M.D.) of lumbar stenosis with right-sided lateral/foraminal compromise attributed to hypertrophy of the yellow ligament. Similar compression may also be due to synovial cysts. Additionally, note the marked hypertrophic changes of the right L4–L5 facet joint
Figure 4
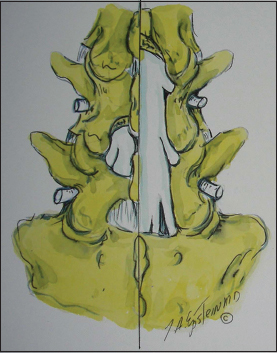
This figure illustrates (Joseph A. Epstein M.D., copyright Nancy E. Epstein M.D.) on the patient’s left side (dorsal view), a focal laminotomy at the L4–L5 level. On the right side you see a partial L3 hemilaminectomy, and full hemilaminectomies at the L4 and L5 levels with medial facetectomies/foraminotomies. The largest number of patients in this series underwent full L3–S1 laminectomies
Figure 5
The illustration (Joseph A. Epstein M.D., copyright Nancy E. Epstein M.D.) on the left shows at L4–L5, marked degenerative spondylolisthesis, severe facet arthrosis, and superior foraminal L4 inferior L5 root compression. On the right, a laminectomy L3–L5 with medial facetectomy/foraminotomy is shown; undercutting preserved the L4–L5 facets
Figure 6
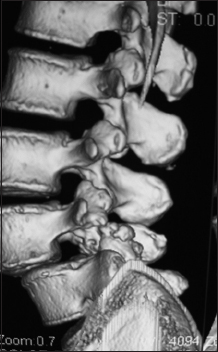
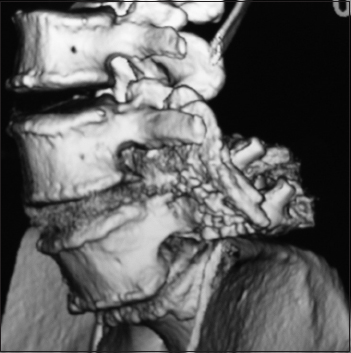
This parasagittal 3D-CT demonstrated a solid posterolateral noninstrumented fusion 6 months postoperatively. Note there is continuity of the bone fragments without intervening lucency of the lamina autograft/Nanoss/BMA fusion mass spanning the L4–L5 transverse processes in a patient with grade I degenerative spondylolisthesis
Figure 7
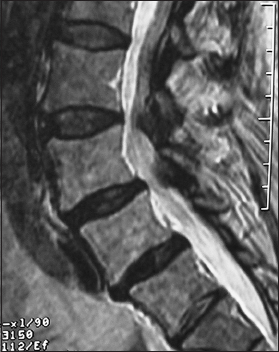
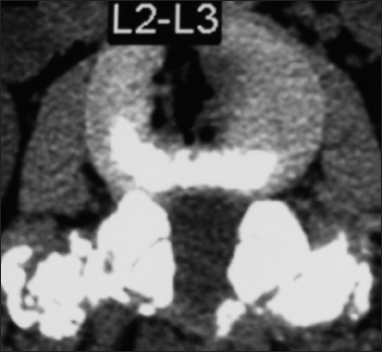
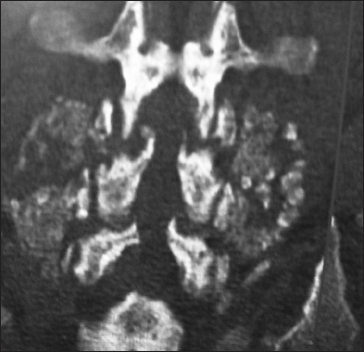
This axial L2-L3 soft tissue window CT demonstrated continuity/fusion of the posterolateral fusion mass and additional arthrodesis across the L2–L3 facet joints. The adequacy of fusion was also checked on the bone window axial CT as the soft tissue study may exaggerate the adequacy of fusion
MATERIALS AND METHODS
Clinical data
Fifty-nine patients, averaging 66.1 years of age, prospectively underwent multilevel lumbar laminectomies with noninstrumented fusions. These procedures utilized lamina autograft (harvested during the decompression) and the bone graft expander Nanoss (RTI Surgical Alachua, FL, and USA) with autogenous BMA [
Surgery
Laminectomy
Lumbar laminectomies were routinely performed through a midline incision, and included medial facetectomy/foraminotomy except for more extensive facet resections warranted with spondylolisthesis/lysis (Gill procedure). More extensive foraminal decompressions also addressed; foraminal/far lateral synovial cysts and foraminal/far lateral discs [Figures
Fusion mass: Lamina autograft and Nanoss/BMA
The fusion mass included all autogenous bone harvested during the laminectomy plus Nanoss/BMA. The Nanoss 10 cm × 2.5 cm strips were impregnated with 10 cc of BMA harvested with dry cottonoids from back-bleeding once the spinous processes were removed with a rib cutter. Prior to application, the strips were cut longitudinally into quarters. For a one-level noninstrumented PLF, two quarters were placed dorsal to the autograft [following prior decortication of the transverse processes (TP)] covering two TPs; for a two-level fusion, typically two strips were used, one on each side covering the three TPs.
Noninstrumented fusion
Noninstrumented PLF fusions required exposure of the TP. This requires adequate muscle/adipose/soft tissue removal over the TP to create a pocket to hold the fusion mass following TP decortication [Figures
RESULTS
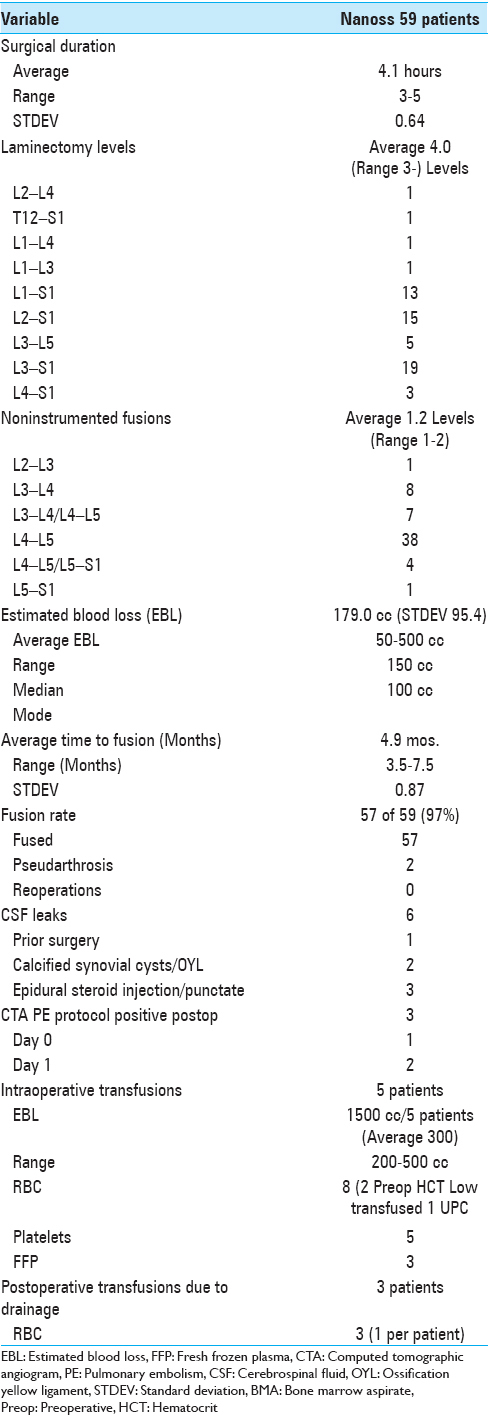
Average 4.0 level laminectomies and 1.2 level noninstrumented PLF fusions were performed requiring an average operative time of 4.1 hours [
Six cerebrospinal fluid leaks (three due to prior epidural injections) did not contribute to postoperative pseudarthroses
Six patients had intraoperative cerebrospinal fluid (CSF) fistulas; 3 were attributed to prior epidural steroid injections (e.g., Tuohy needle holes), 2 had calcified synovial cysts extending through the dura, and 1 had marked scar due to prior surgery. All 6 were primarily repaired in a watertight fashion using 7-0 Gore-Tex sutures (Gore Medical, Flagstaff, AZ, USA), muscle patch grafts for the latter 3, and Duragen in all cases (Integra Surgical, Hawthorne, NY, USA) [
No postoperative infections with lamina autograft and Nanoss/BMA
None of the 59 patients undergoing multilevel lumbar laminectomies with noninstrumented PLF using lamina autograft and Nanoss/BMA developed a postoperative infection. The prevention of infection was largely attributed to the use of Hibiclens washes started 2 weeks preoperatively, the intraoperative use of antibiotic irrigation every 15 minutes, the routine use of postoperative prophylactic antibiotics, and employing Silverlon dressings for up to one postoperative month.
Postoperative pulmonary emboli requiring 6-week delayed full-dose Lovenox did not correlate with postoperative pseudarthroses
None of the 3 patients who developed pulmonary emboli (PE) documented on computed tomographic angiography (CTA)-PE protocols the night of surgery (1 patient) and on postoperative day 1 (2 patients) [
Postoperative pseudarthrosis did not correlate with intraoperative or postoperative transfusions
None of the 5 patients who required intraoperative or 3 patients who required postoperative transfusions later developed pseudarthroses [
DISCUSSION
Prior documentation of the efficacy of bone graft expander Vitoss/BMA for lumbar instrumented and noninstrumented posterolateral fusions
Previously, Epstein documented the efficacy of Vitoss/BMA as a bone graft expander for PLF. In 2006, Vitoss/BMA and lamina autograft (50:50 mix) were utilized to perform 40 laminectomies (average 3.7 levels), and one (27 patients) and two (13 patients) level posterolateral instrumented pedicle/screw/rod fusions.[
Bone graft extender Nanoss/BMA
Nanoss was approved by the Food and Drug Administration (FDA) in 2008 as a bone void filler/extender for posterolateral spinal fusions (e.g., including lumbar fusions). It is a nanostructured hydroxyapatite (HA), with an engineered extracellular osteoconductive bioscaffold matrix that facilitates cell infiltration. When lamina autograft and BMA are added, it becomes not only osteoconductive, but also osteoinductive and osteogenic.
Comparable efficacy of Vitoss vs. Nanoss/BMA as a bone graft extender for noninstrumented lumbar PLF
In 2015, Epstein documented the comparable safety/efficacy of lamina autograft with Vitoss/BMA (213 patients) vs. Nanoss/BMA (45 patients) for posterolateral lumbar noninstrumented PLF.[
Safety/Efficacy of lamina autograft and Nanoss/BMA alone for noninstrumented lumbar PLF
In this series, patients underwent average 4.0-level lumbar laminectomies/average 1.2 level noninstrumented PLF fusions using lamina autograft and Nanoss/BMA. An average of 4.9 months postoperatively, both dynamic X-rays and CT studies documented a 97% (57 of 59) fusion rate. Two patients had radiographic pseudarthrosis; both were severely osteoporotic, morbidly obese, and were active smokers; neither required secondary surgery. No Nanoss-related complications such as infections, seromas, and or hematomas were observed. Note the higher fusion rates seen in the more recent studies with both Vitoss/BMA (older study 85%, newer study 98.6%) and Nanoss/BMA (100% and 97%) may have in part been attributed to better operative technique learned over time by the same surgeon.
Efficacy of demineralized bone matrix/inductive conductive matrix (DBM/ICM: Medtronic, Memphis, TN, USA) to supplement lamina autograft for multilevel laminectomy and noninstrumented posterolateral fusion
In 2008, Epstein evaluated fusion rates for 75 patients (average age 69) undergoing average 4.9-level lumbar laminectomies and average 2.0-level noninstrumented PLF utilizing lamina autograft with demineralized bone matrix/ICM (50:50 mix autograft: DBM/ICM).[
Other bone graft expanders
Bone morphogenetic protein (BMP: INFUSE, Medtronic, Memphis, TN, USA), other DBM, and ceramics
Other bone graft expanders, including bone morphogenetic protein (BMP: INFUSE, Medtronic, Memphis, USA)), other demineralized bone matrix (DBM) products, and ceramics have been utilized alone or in combination with autograft to promote spinal fusion. Although BMP promoted fusion even when used alone as a bone graft substitute, increasingly, there were major concerns about its complications (e.g., heterotopic ossification, osteolysis, postoperative seromas, increased infection, and increased cancer rates).[
Actifuse (Baxter Corporation, Franklin Lakes, NJ, USA, Deerfield Il, USA)
Actifuse was another bone graft extender used for posterolateral spinal fusions.[
CONCLUSION
Multiple bone graft extenders/supplements/substitutes, including BMP/INFUSE, DMB/ICM, DBM, Actifuse, Vitoss, and now Nanoss/BMA have been utilized to perform instrumented and noninstrumented lumbar posterolateral fusions. Here, we prospectively performed 59 average 4.0-level laminectomies and average 1.2-level noninstrumented fusions in patients with severe osteoporosis and/or obesity (morbid obesity) utilizing lamina autograft and Nanoss/BMA. Pathology contributing to instability included degenerative spondylolisthesis (51 patients)/lysis (2 patients), and/or foraminal/far lateral synovial cysts (32 patients) and/or discs (21 patients) warranting partial/full facetectomies. Both dynamic X-ray and CT studies documented a 97% fusion (57 of 59 patients) rate an average of 4.9 months postoperatively; 2 pseudarthroses were attributed to severe osteoporosis, morbid obesity, and smoking. As no other Nanoss-related complications occurred, this study confirms the safety/efficacy of Nanoss/BMA as a bone graft extender for noninstrumented PLF.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Baumann F, Krutsch W, Pfeifer C, Neumann C, Nerlich M, Loibl M. Posterolateral fusion in acute traumatic thoracolumbar fractures: A comparison of demineralized bone matrix and autologous bone graft. Acta Chir Orthop Traumatol Cech. 2015. 82: 119-25
2. Epstein NE. A preliminary study of the efficacy of Beta Tricalcium Phosphate as a bone expander for instrumented posterolateral lumbar fusions. J Spinal Disord Tech. 2006. 19: 424-9
3. Epstein NE. Efficacy of different bone volume expanders for augmenting lumbar fusions. Surg Neurol. 2008. 69: 16-9
4. Epstein NE. An analysis of noninstrumented posterolateral lumbar fusions performed in predominantly geriatric patients using lamina autograft and beta tricalcium phosphate. Spine J. 2008. 8: 882-7
5. Epstein NE. Fusion rates and SF-36 outcomes after multilevel laminectomy and noninstrumented lumbar fusions in a predominantly geriatric population. J Spinal Disord Tech. 2008. 21: 159-64
6. Epstein NE. Preliminary study showing safety/efficacy of nanoss bioactive versus vitoss as bone graft expanders for lumbar noninstrumented fusions. Surg Neurol Int. 2015. 6: S318-22
7. Grabowski G, Cornett CA. Bone graft and bone graft substitutes in spine surgery: Current concepts and controversies. J Am Acad Orthop Surg. 2013. 21: 51-60
8. Kadam A, Millhouse PW, Kepler CK, Radcliff KE, Fehlings MG, Janssen ME. Bone substitutes and expanders in Spine Surgery: A review of their fusion efficacies. Int J Spine Surg. 2016. 10: 33-
9. Lerner T, Liljenqvist U. Silicate-substituted calcium phosphate as a bone graft substitute in surgery for adolescent idiopathic scoliosis. Eur Spine J. 2013. 22: S185-94
10. Licina P, Coughlan M, Johnston E, Pearcy M. Comparison of Silicate-Substituted Calcium Phosphate (Actifuse) with Recombinant Human Bone Morphogenetic Protein-2 (Infuse) in Posterolateral Instrumented Lumbar Fusion. Global Spine J. 2015. 5: 471-8






Ralph E. Rydell M.D. M.S.
Posted August 1, 2017, 12:58 pm
I greatly appreciate the clarity of description of your technique, the succinct review of the fusion matrix options, and admire your results. Emphasis on meticulous TP and lateral facet decortication is , I think, also very important in achieving these results.
nancy Epstein MD Editor in Chief SNI
Posted August 2, 2017, 4:29 am
Dear Dr. Rydell
Thank you for your comments. I truly believe this less invasive fusion method really works and is so much safer for the older patients esp. those with marked osteoporosis. Again, thank you for taking the time to comment.