- Chief of Neurosurgical Spine/Education, NYU Winthrop Hospital, Mineola, New York, USA
Correspondence Address:
Nancy E. Epstein
Chief of Neurosurgical Spine/Education, NYU Winthrop Hospital, Mineola, New York, USA
DOI:10.4103/sni.sni_241_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy E. Epstein. High posterior cervical fusion rates with iliac autograft and Nanoss/bone marrow aspirate. 20-Jul-2017;8:152
How to cite this URL: Nancy E. Epstein. High posterior cervical fusion rates with iliac autograft and Nanoss/bone marrow aspirate. 20-Jul-2017;8:152. Available from: http://surgicalneurologyint.com/surgicalint-articles/high-posterior-cervical-fusion-rates-with-iliac-autograft-and-nanossbone-marrow-aspirate/
Abstract
Background:Patients with severe cervical multilevel stenosis and an adequate lordotic curvature often undergo multilevel laminectomies with posterior instrumented fusions. Although the “gold standard” for the fusion mass remains iliac crest autograft, many require additional volume provided by bone graft expanders. Here, we studied the fusion rates for 32 patients undergoing multilevel cervical laminectomy and vertex/rod/eyelet/titanium cable fusions utilizing lamina/iliac autograft and the bone graft expander Nanoss (RTI Surgical, Alachua, FL, USA) with autogenous bone marrow aspirate (BMA).
Methods:Thirty-two patients, averaging 63.0 years of age, presented with severe cervical myeloradiculopathy (average Nurick Grade 4.4). Magnetic resonance (MR) studies documented 2–3-level high intrinsic cord signals, whereas computed tomography (CT) scans confirmed marked stenosis and ossification of the posterior longitudinal ligament (OPLL)/ossification of the yellow ligament (OYL). Patients underwent multilevel lamnectomies/instrumented fusions, and were followed up for an average of 2.7 years.
Results:Multilevel laminectomies (2.8 levels) and average 7.8-level vertex/rod/eyelet/cable fusions were performed utilizing lamina/iliac crest autograft and Nanoss/BMA. Fusion was confirmed on X-ray/CT studies an average of 4.7 months postoperatively in 31 of 32 patients (97%); there was just one pseudarthrosis requiring secondary surgery. The only other complication was a delayed transient C5 palsy that fully resolved in 6 postoperative months.
Conclusions:Thirty-two severely myelopathic underwent 2.8-level cervical laminectomies/7.8 level fusions utilizing lamina/iliac autograft and Nanoss/BMA. Fusion was documented on both dynamic X-ray and CT studies in 31 of 32 (97%) patients an average of 4.7 months postoperatively. Nanoss/BMA appears to be a safe and effective bone graft expander that can be utilized for posterior cervical fusions.
Keywords: Bone graft expander, bone marrow aspirate (BMA), high fusion rates, Nanoss, posterior cervical fusions
INTRODUCTION
Following multilevel cervical laminectomies/posterior fusions, the volume of iliac crest autograft (e.g., the “gold standard”) may require supplementation with a bone graft expander. Although there are many types available here we prospectively performed 32 multilevel level cervical laminectomies/posterior instrumented fusions (vertex/rod/eyelet/titanium cable) utilizing lamina/iliac crest autograft supplemented with Nanoss (RTI Surgical Alachua, FL, USA) and autologous bone marrow aspirate (BMA). Over an average of 2.7 postoperative years, we utilized both dynamic X-ray and computed tomography (CT) studies to assess fusion rates.
MATERIALS AND METHODS
Prospectively, 32 patients averaging 63 years of age presented with severe cervical myeloradiculopathy (average Nurick Grade 4.4) [
Figure 1
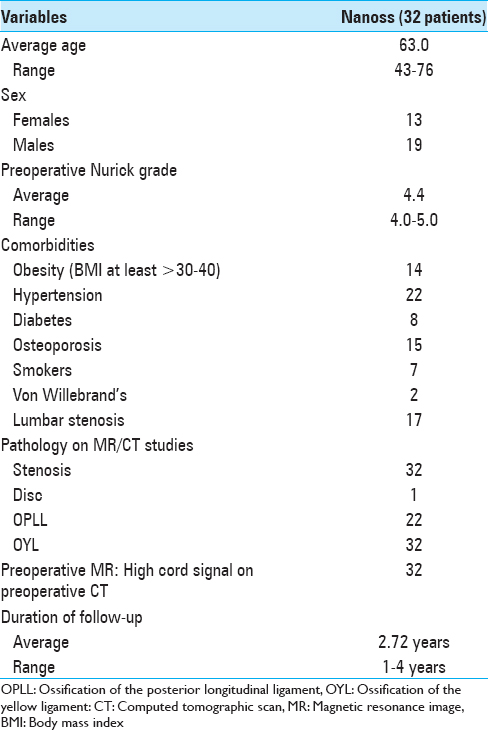
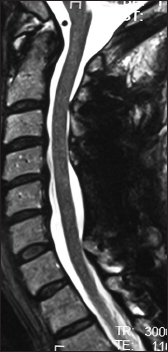
This preoperative cervical T2-weighted sagittal midline MR documented posterolateral cord compression at the C3–C4 level and C6–C7 levels. Here, lamienctomies at C3 and C6/C7 resulted in sufficient posterolateral cord decompression with resection of ossification of the yellow ligament and decompression of dorsolateral shingling of the respective laminae. Here, laminectomy of C3, C6, and C7 with C2–T2 posterior fusion resulted in adequate cord decompression
Figure 3
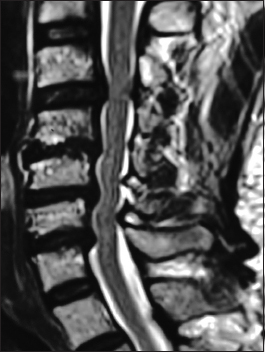
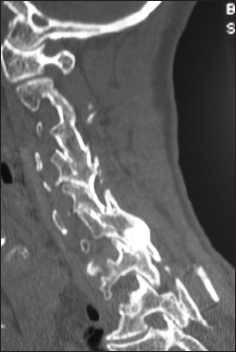
The preoperative soft tissue sagittal 2D-CT documented marked spinal stenosis at the C3–C4 level attributed to anterior ossification of the posterior longitudinal ligament (OPLL) and dorsolateral inward shingling/stenosis involving the C3 and C4 laminae. Here, a laminectomy of C3, C4 with posterior fusion C2–C5/C6 adequately decompressed the spinal cord
Figure 4
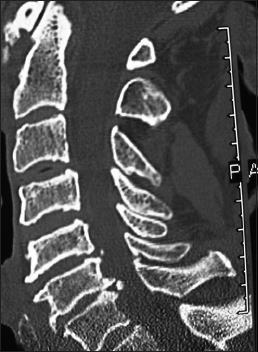
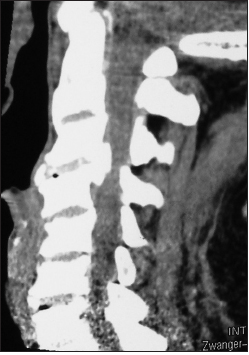
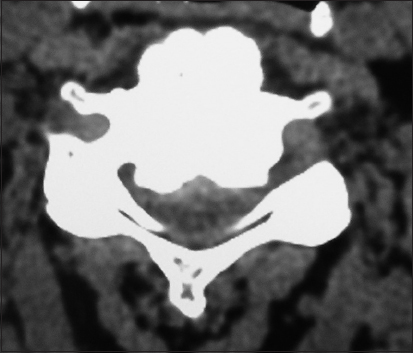
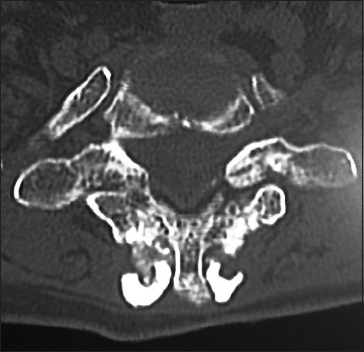
On this preoperative axial noncontrast CT study obtained at the C5–C6 level, there is marked ventral ossification of the posterior longitudinal ligament accompanied by dorsolateral inward shingling of both the C5 and C6 laminae (note both laminae are seen on the same image posteriorly). The combined pathology reduced the AP diameter of the spinal canal to less than 6 mm
Figure 5
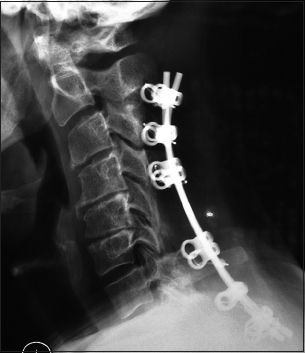
The postoperative plain X-ray documented the laminectomy defects at the C5, C6 levels, and the posterior vertex/rod/eyelet/titanium cable system applied to the spinous processes of C2, C3, C4, C7, T1, and T2. Note there was some pullout of C2 but the remaining cables remained in place until the patient fused without the need for further surgery
Fusion technique/mass: Vertex/rod/eyelet fusion, autograft, and Nanoss/BMA
The vertex/rod/eyelet/titanium cable system was applied to the intact spinous processes cephalad/caudad to the laminectomy levels [
RESULTS
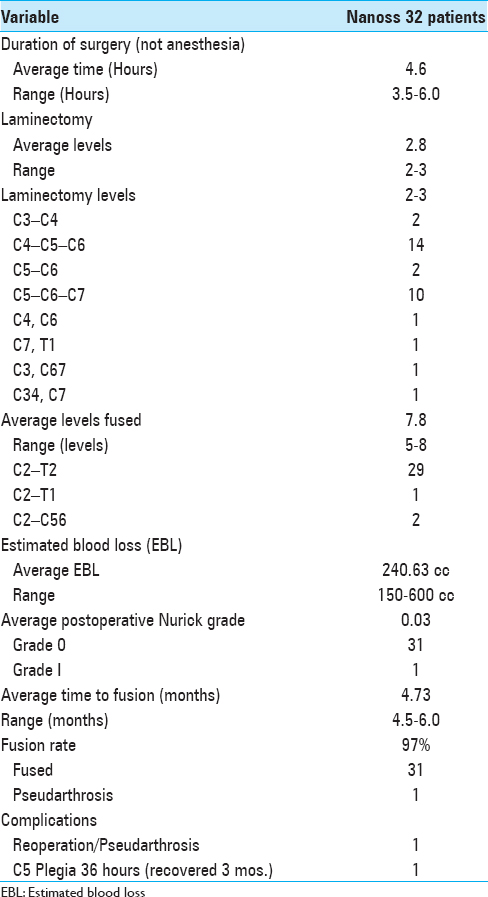
Cervical surgery required an average of 4.6 hours. Patients underwent average 2.8-level cervical laminectomies/7.8-level posterior fusions utilizing lamina/iliac crest autograft and Nanoss/BMA [
Avoidance of infections
No patient developed an infection. The avoidance of infections was largely attributed to the use of Hibiclens washes started 2 weeks preoperatively, the intraoperative use of antibiotic irrigation every 15 minutes, the use of postoperative prophylactic antibiotics, and utilizing a Silverlon dressing for up to 1 month postoperatively on the posterior cervical wound.
DISCUSSION
Iliac crest autograft (the “gold standard”) with Nanoss/BMA
Iliac crest autograft is still considered the “gold standard” for a fusion mass. Nevertheless, when the autograft fusion mass is insufficient, Nanoss provides a Food and Drug Administration (FDA) approved bone void filler/expander (e.g., approved for posterolateral spinal fusions) when combined with laminar/iliac autograft and BMA. Nanoss, composed of nanostructured hydroxyapatite (HA), is an engineered extracellular osteoconductive bioscaffold matrix that facilitates cell infiltration. In this study, we confirmed the efficacy of Nanoss/BMA in promoting a 97% posterior cervical fusion rate. In several prior studies, Nanoss was found to be comparable to Vitoss (Stryker, Kalamazoo, MI, USA).
Safety/efficacy of Vitoss, and Vitoss vs. Nanoss in Lumbar Spine Fusion
The safety and efficacy of Vitoss and Vitoss vs. Nanoss as bone graft expanders were documented in prior studies of the lumbar spine. In 2006, Epstein utilized Vitoss and lamina autograft (50:50 mix) to perform 40 laminectomies (average 3.7 levels), and 1 (27 patients) and 2 (13 patients) level posterolateral instrumented pedicle/screw fusions.[
Safety/efficacy of Vitoss and Vitoss vs. Nanoss in Cervical Spine Fusion
The safety and efficacy of Vitoss and Vitoss vs. Nanoss as bone graft expanders were also previously documented in the cervical spine. In 2008, Epstein evaluated the fusion rates for 35 severely myelopathic adults (mean Nurick Grade 4.1) undergoing average 2-level laminectomies with average 7-level posterior cervical vertex/rod eyelet/braided titanium cable fusions utilizing lamina/iliac autograft and Vitoss/BMA.[
Other bone graft expanders
Bone morphogenetic protein, demineralized bone matrix, and ceramics
Other bone graft expanders/supplements, including bone morphogenetic protein [BMP: Infuse; Medtronic, Memphis, USA)], demineralized bone matrix (DBM), and ceramics, have promoted spinal fusions. BMP promoted fusions even without autograft; however, several authors were concerned about observed/anticipated complications (e.g. heterotopic ossification, osteolysis, postoperative seromas, increased infection, and increased cancer rates).[
Actifuse (Baxter Corporation, Franklin Lakes, NJ, USA, Deerfield Il, USA)
Actifuse is another bone graft substitute/extender used in spine surgery. In Lerner and Liljenqvist study in 2013, Actifuse (Si-CaP) was combined with BMA to provide an adequate fusion mass for adolescent idiopathic scoliosis surgery (AIS).[
CONCLUSION
Multiple bone graft expanders (e.g., BMP, DBM, Vitoss, Actifuse, and now Nanoss) have been trialed to supplement and/or occasionally supplant iliac crest bone graft. In this study, Nanoss/BMA combined with iliac/laminar autograft resulted in a 97% posterior cervical fusion rate in 31 of 32 patients, confirmed on both dynamic X-ray and CT studies an average of 4.7 months postoperatively. Notably, other than the one pseudarthrosis, there were no Nanoss-related complications. These preliminary data appear to confirm the safety/efficacy of Nanoss for posterolateral cervical spine fusions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Baumann F, Krutsch W, Pfeifer C, Neumann C, Nerlich M, Loibl M. Posterolateral fusion in acute traumatic thoracolumbar fractures: A comparison of demineralized bone matrix and autologous bone graft. Acta Chir Orthop Traumatol Cech. 2015. 82: 119-25
2. Epstein NE. A preliminary study of the efficacy of Beta Tricalcium Phosphate as a bone expander for instrumented posterolateral lumbar fusions. J Spinal Disord Tech. 2006. 19: 424-9
3. Epstein NE. Efficacy of different bone volume expanders for augmenting lumbar fusions. Surg Neurol. 2008. 69: 16-9
4. Epstein NE. An argument for traditional posterior cervical fusion techniques: Evidence from 35 cases. Surg Neurol. 2008. 70: 45-51
5. Epstein NE. Efficacy of posterior cervical fusions utilizing an artificial bone graft expander, beta tricalcium phosphate. Surg Neurol Int. 2011. 2: 15-
6. Epstein NE. Preliminary documentation of the comparable efficacy of vitoss versus NanOss bioactive as bone graft expanders for posterior cervical fusion. Surg Neurol Int. 2015. 6: S164-71
7. Epstein NE. Preliminary study showing safety/efficacy of nanoss bioactive versus vitoss as bone graft expanders for lumbar noninstrumented fusions. Surg Neurol Int. 2015. 6: S318-22
8. Fredericks D, Petersen EB, Watson N, Grosland N, Gibson-Corley K, Smucker J. Comparison of Two Synthetic Bone Graft Products in a Rabbit Posterolateral Fusion Model. Iowa Orthop J. 2016. 36: 167-73
9. Grabowski G, Cornett CA. Bone graft and bone graft substitutes in spine surgery: Current concepts and controversies. J Am Acad Orthop Surg. 2013. 21: 51-60
10. Kadam A, Millhouse PW, Kepler CK, Radcliff KE, Fehlings MG, Janssen ME. Bone substitutes and expanders in Spine Surgery: A review of their fusion efficacies. Int J Spine Surg. 2016. 10: 33-
11. Lerner T, Liljenqvist U. Silicate-substituted calcium phosphate as a bone graft substitute in surgery for adolescent idiopathic scoliosis. Eur Spine J. 2013. 22: S185-94
12. Licina P, Coughlan M, Johnston E, Pearcy M. Comparison of Silicate-Substituted Calcium Phosphate (Actifuse) with Recombinant Human Bone Morphogenetic Protein-2 (Infuse) in Posterolateral Instrumented Lumbar Fusion. Global Spine J. 2015. 5: 471-8