- Department of Neurosurgery, Fukuoka Children’s Hospital, Fukuoka, Japan
- Department of Psychiatry, Shourai Hospital, Karatsu, Japan
- Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Iizuka, Japan
- Department of Neurosurgery, Iizuka Hospital, Iizuka, Japan
- Department of Neurosurgery, Hachisuga Hospital, Fukuoka, Japan.
Correspondence Address:
Nobuya Murakami, Department of Neurosurgery, Fukuoka Children’s Hospital, Fukuoka, Japan.
DOI:10.25259/SNI_989_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nobuya Murakami1, Ai Kurogi1, Satoshi O. Suzuki2, Takafumi Shimogawa3, Nobutaka Mukae4, Koji Yoshimoto3, Takato Morioka5. Histopathological presence of dermal elements in resected margins of neural structures obtained from initial repair surgery for myelomeningocele. 06-Jan-2023;14:7
How to cite this URL: Nobuya Murakami1, Ai Kurogi1, Satoshi O. Suzuki2, Takafumi Shimogawa3, Nobutaka Mukae4, Koji Yoshimoto3, Takato Morioka5. Histopathological presence of dermal elements in resected margins of neural structures obtained from initial repair surgery for myelomeningocele. 06-Jan-2023;14:7. Available from: https://surgicalneurologyint.com/surgicalint-articles/12092/
Abstract
Background: Development of dermoid or epidermoid cysts in myelomeningocele (MMC) sites is generally thought to occur in a delayed fashion due to implantation of dermal elements during initial repair surgery. Another theory is that dermal and dermoid elements may already be present within dysplastic neural structures at birth.
Methods: We experienced histopathological presence of dermal elements in resected tissues at initial repair surgery in four out of 18 cases with MMC who required resection of parts or margins of the neural structures to perform cord untethering. Since one of these cases has already been reported, we describe the clinicopathological findings for the remaining three cases.
Results: In Case1, cryptic dermoid elements were discovered in the terminal filum-like structure (FT-LS) caudal to the open neural placode (NP). The FT-LS had histopathological characteristics similar to the retained medullary cord. In Case 2, dermoid elements were discovered in the caudal margin of the dysplastic conus medullaris. In Case 3, a thin squamous epithelial layer overlapped the rostral margin of the NP where the NP was located near the skin. Case 1 developed an epidermoid cyst at 1 year and 2 months of age, which was totally resected.
Conclusion: Prenatally existing cryptic dermoid elements in the caudal portion of neural structures and remnants of dermal elements overlapping the rostral margin of the NP are associated with delayed occurrence of dermoid/ epidermoid cysts. Postoperative histopathological investigation of the resected specimens is recommended. Once dermal elements are revealed, repeated imaging examination and additional surgery should be considered.
Keywords: Dermoid/epidermoid cyst, Inclusion tumor, Keratin, Retained medullar cord, Squamous epithelium
INTRODUCTION
Dermoid or epidermoid cysts and related spinal cord tethering are one of the serious complications that may develop in patients who have undergone previous repair surgery for myelomeningocele (MMC) during the neonatal period.[
MATERIALS AND METHODS
From January 2018 to September 2022, 24 patients with MMC underwent initial repair surgery at Fukuoka Children’s Hospital. At surgery, an open (dysplastic) NP, that is myeloschisis, or a dysplastic CM was resected at the intermediate zone which consists of pia-arachnoid membrane between the neural structure and the surrounding skin[
Of the 24 MMC patients, 18 patients required resection of parts or margins of the neural structures to perform complete untethering of the cord. The resected tissues were placed in formalin. Routine histopathological sections were performed with hematoxylin and eosin (H&E) staining and immunostaining such as glial fibrillary acidic protein (GFAP) for neuroglial tissues and CK 5/6 and CK14 for cytokeratin as part of standard diagnostic analysis.
RESULTS
In the resected specimens of 4 (22%) out of 18 patients, histopathological examination revealed dermal or dermoid elements. Since we have already reported one of the four cases in detail,[
CASE REPORTS
Case 1
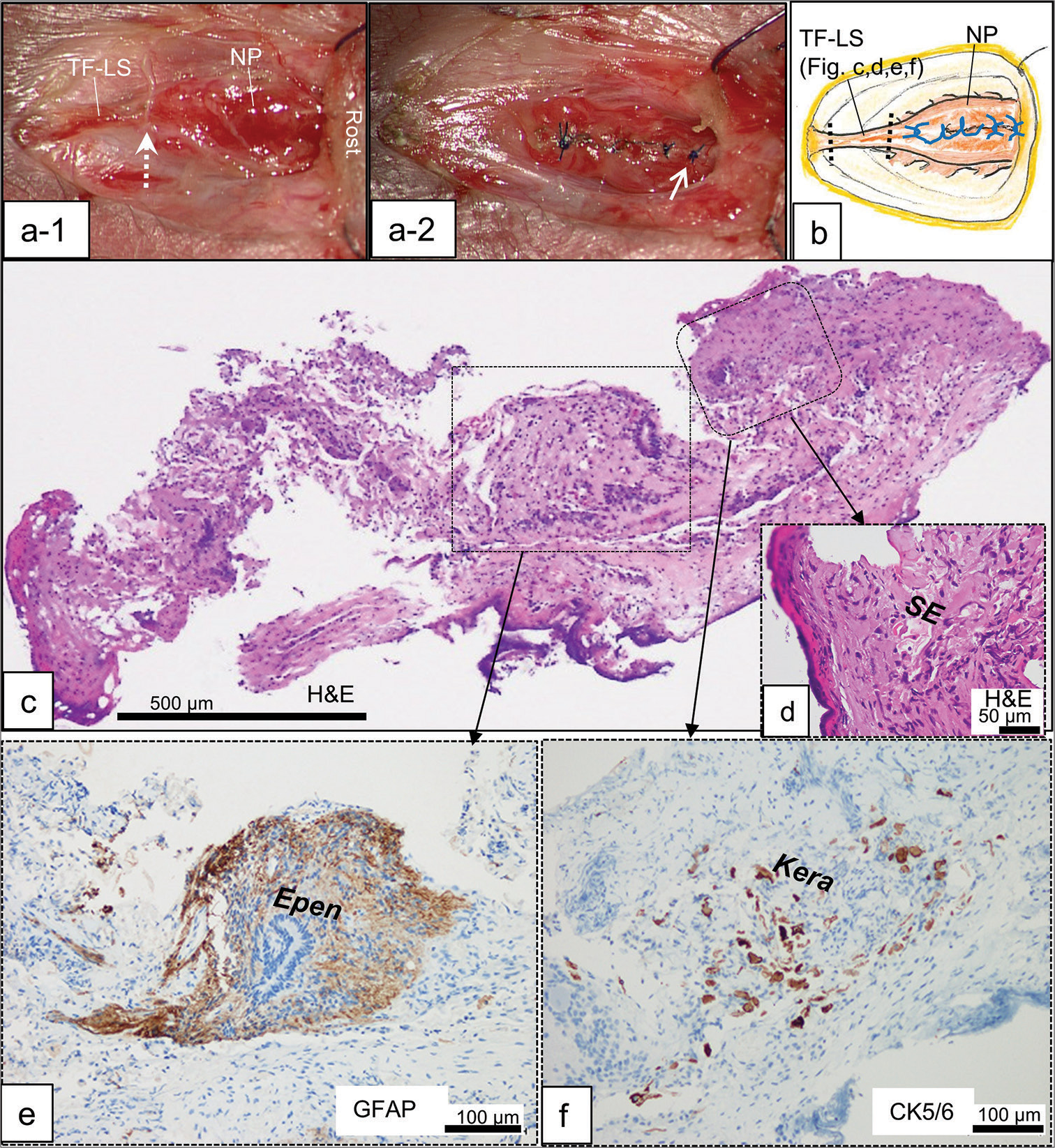
Case 1 was a female infant born prematurely at 26 weeks and 2 days and weighing 784 g. She exhibited an open NP in the open spinal canal at the lumbosacral level. She had slight impairment in plantar flexion and in anal function. Repair surgery for MMC 1 day after birth revealed a TF-LS caudal to the NP [
Figure 1:
Case 1: First surgery (a-1) intraoperative photograph of the open neural placode (NP) and terminal filum-like structure (TF-LS) in the open spinal canal. The TF-LS is severed at the border to the neural placode (dotted arrow). Rost: rostral side. (a-2) Reconstituted neural placode with pia-arachnoidal suture using unabsorbable monofilament. Note the location of the most rostral knot (arrow). (b) Schematic drawing of the surgery showing the relationship between the severed lines of TF-LS (dotted lines) and the knots (blue lines). Histopathological findings of the transverse sections of the TF-LS stained with hematoxylin and eosin (H&E; c and d), and immunostained with glial fibrillary acidic protein (GFAP; e) and cytokeratin marker CK5/6 (f). Higher magnification views of the area are indicated by dashed squares in (c). The specimen composed of GFAP immunopositive neuroglial tissues with ependymal canal-like structure (Epen: e) abutting on a fibrocollagenous tissue. Squamous epithelium (SE; d), and CK5/6 immunopositive keratinizing cells associated with keratin debris (Kera; f) are focally embedded inside of the fibrocollagenous tissue.
Histopathologically, the severed TF-LS consisted of GFAP immunopositive neuroglial tissues with ependymal canal abutting on a fibrocollagenous tissue [
We repeated follow-up MRI, and 3D-hT2WI at 2 months after surgery confirmed successful untethering [
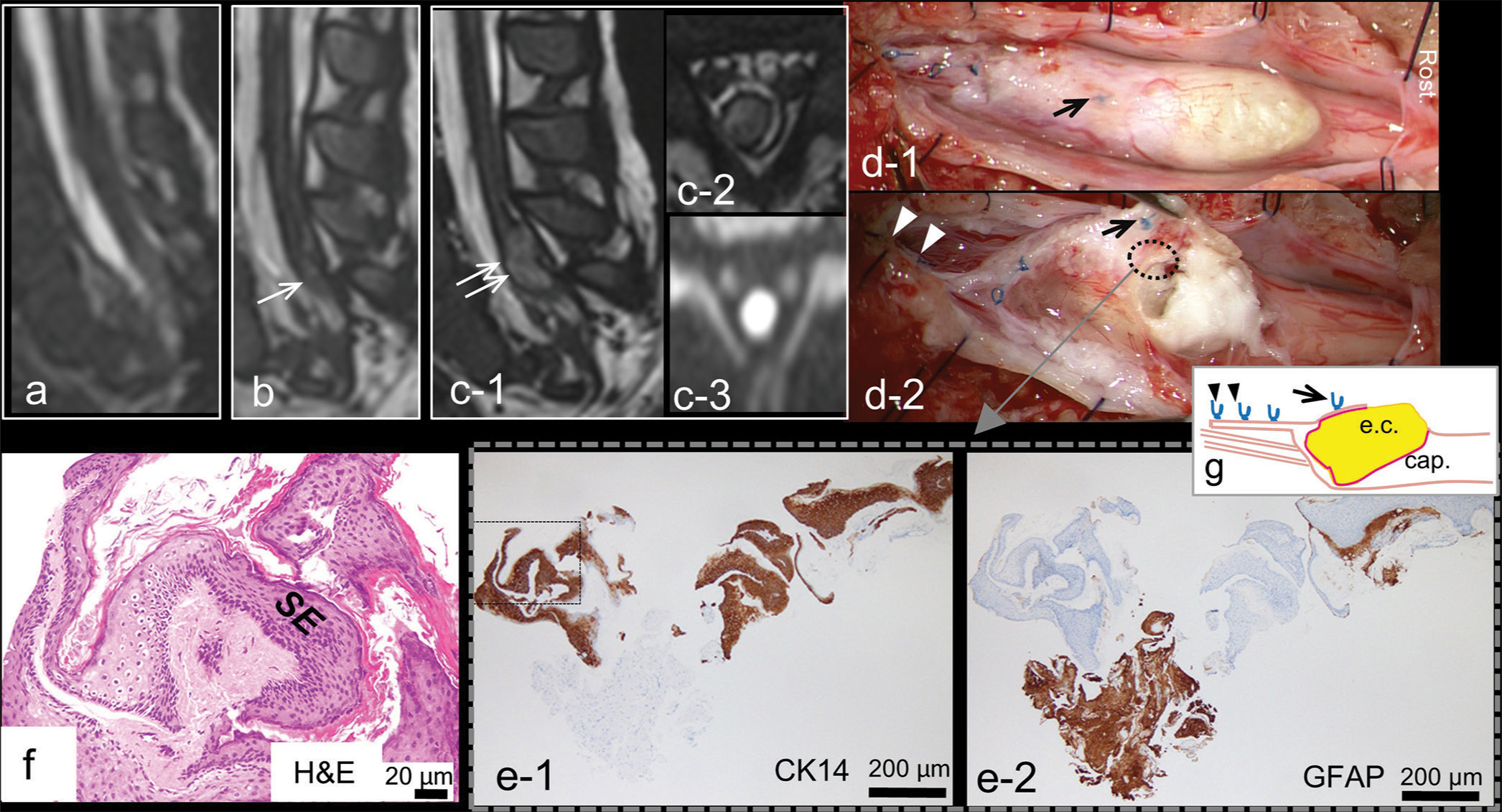
Figure 2:
Case 1: Second surgery (a-c) follow-up 3D-heavily T2-weighted magnet resonance imaging in chronological order. (a) A sagittal image at 2 months after the first surgery showing reconstituted spinal cord in the dural cul-de-sac. (b) An image at 1 year and 2 months of age demonstrating small hyperintense lesion (arrow) in the caudal end of the spinal cord. Sagittal (c-1) and axial (c-2) images at 2 years and 6 months of age revealing mass lesion (double arrow; c-1) with strong hyperintensity on diffusion-weighted image (c-3), indicating a dermoid or epidermoid cyst. (d-1) Intraoperative photograph showing a pearly tumor in the conus medullaris (CM) with partial exophytic extension, located rostrally to the most rostral knot (arrow) used in the first surgery. Rost: rostral side. (d-2) Opening of the cyst revealing the tumor capsule and keratin substance located around and rostral to the most rostral knot (arrow), but not in the vicinity of the caudal knots (arrowheads). Histopathological findings of the resected capsule indicated by dotted ellipse in (d-2) immunostained with CK14 (e-1) and glial fibrillary acidic protein (GFAP) (e-2). (f) Higher magnification view of the area indicated by dashed square in (e-1) stained with H&E. Stratified squamous epithelium (SE; f) which is immunopositive for CK14 (e-1), associated with keratin debris but without hair shafts or skin appendage, are abutted on a GFAP immunopositive gliotic central nervous tissue (e-2). (g) Schematic drawing of the CM from a lateral view indicating the epidermoid cyst (e.c.) with capsule (cap.) beneath the most rostral knot (arrow) and the absence of epidermoid cyst in the vicinity of the caudal knots (arrow heads).
Laminoplastic laminotomy of L3-4 revealed a pearly tumor in the CM with partial exophytic extension. Opening of the cyst revealed the tumor capsule and keratin substance existing around and rostral to the most rostral knot of unabsorbable monofilament used in the first surgery, but not in the vicinity of the caudal knots, where the morphological border to the filum terminale had been at the first surgery [
Histopathologically, the capsule consisted of stratified squamous epithelium associated with keratin debris, but without hair shafts or skin appendage, abutting on a gliotic central nervous tissue, and the diagnosis of epidermoid cyst were made [
Case 2
Case 2 was a girl who had a cord-like structure in the open spinal canal, which appeared as dysplastic CM [
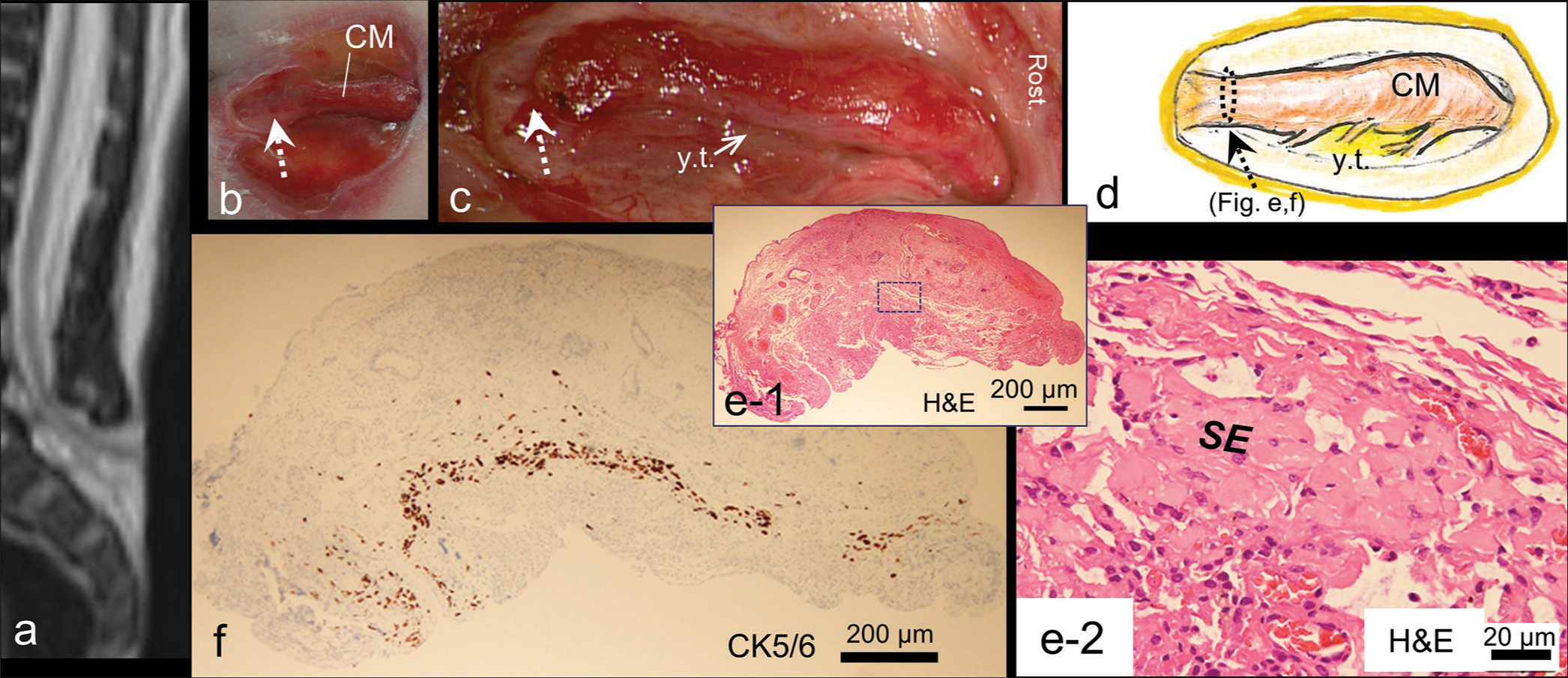
Figure 3:
Case 2 (a) 3D-heavily T2-weighted image at birth indicating a neural structure extending dorsally from the spinal cord. Intraoperative photograph (b and c) and schematic drawing (d) of the dysplastic conus medullaris (CM) in the open spinal canal. Rost: rostral side. The caudal resection line of the CM (dotted arrow in (b-d) is located at its most rostral non-functional portion, where the border to the skin is blurred. After untethering of the CM (c), yellowish viscid tissues (y.t. in [c and d]) are observed between the nerve roots. Histopathological findings of the resected caudal margin of the CM stained with H&E (e-1 and 2) and immunostained with CK5/6 (f), of which location is indicated by dashed ellipse in (d). Higher magnification view of the area is indicated by dashed square in (e-1). Squamous epithelium cells (SE: e-2), which are CK5/6 immunopositive (f), are focally embedded in the fibrocollagenous tissues.
Histopathologically, the resected caudal margin of the CM composed of fibrocollagenous tissues focally embedded with arachnoid cells and CK5/6 and CK14 immunopositive squamous epithelial cells [
She underwent a ventricle-peritoneal shunt at 6 months of age due to hydrocephalus. Follow-up MRI performed at 1 year and 6 months of age demonstrated absence of the dermoid cysts at the lumbosacral region.
Case 3
Case 3 was a male infant who exhibited a NP in the open spinal canal [
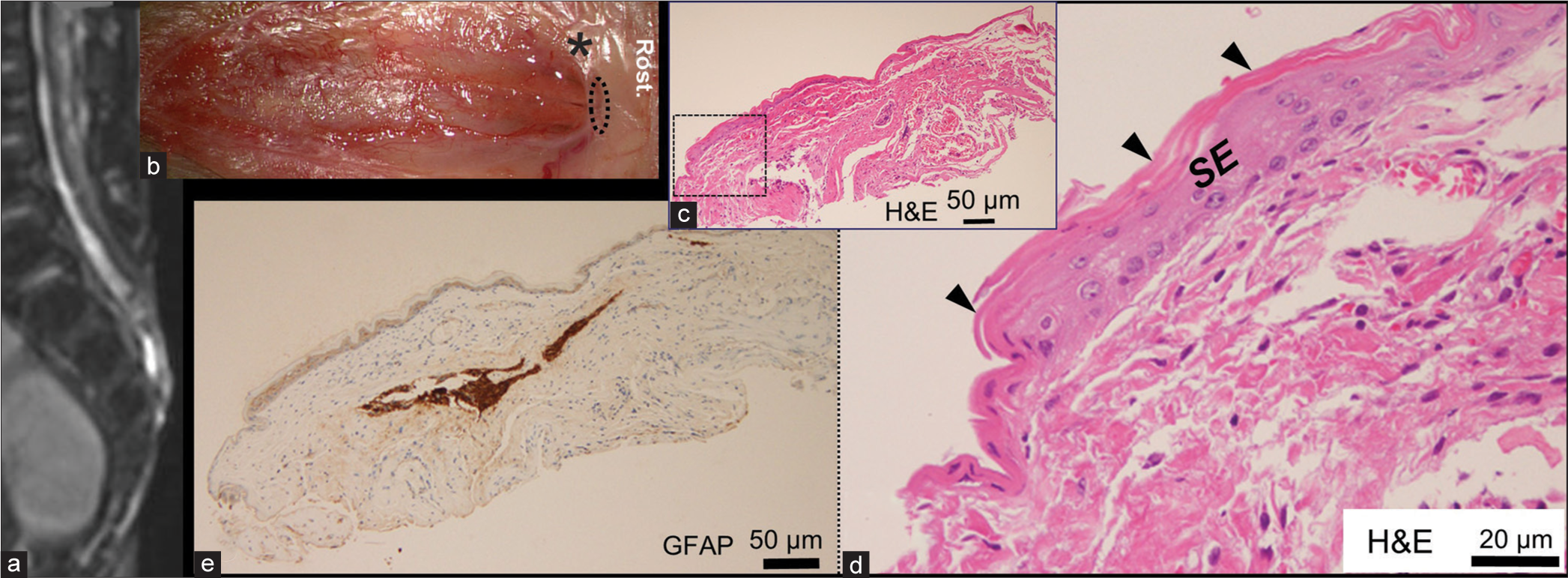
Figure 4:
Case 3 (a) 3D-heavily T2-weighted image at birth indicating a neural placode at the sacral level. (b) Intraoperative photograph of the open neural placode. Rost: rostral side. The rostral portion of the neural placode is located in close proximity to the dysplastic skin layer (*). Histopathological findings of the resected rostral margin of the neural placode, of which location is indicated by dotted ellipse in (b), stained with H&E (c and d) and immunostained with glial fibrillary acidic protein (GFAP) (e). Higher magnification view (d) of the area is indicated by dashed square in (c). Stratified squamous epithelium (SE; d) overlapped by keratinizing layer (arrowheads; d) partly lines fibrocollagenous tissue embedding a small GFAP immunopositive neuroglial tissue (e).
Histopathologically, the resected tissue of rostral margin of the NP consisted of a fibrocollagenous tissue partly lined by keratinizing stratified squamous epithelium, embedding a small neuroglial tissue immunopositive for GFAP [
Follow-up MRI performed at 1 year and 6 months of age showed the absence of dermoid cysts.
DISCUSSION
In Cases 1 and 2, dermoid elements were embedded in the TF-LS caudal to the NP and in the caudal margin of the dysplastic CM, respectively, suggesting prenatally existing cryptic dermoid elements. The origin of the dermoid element located inside these structures might be prenatal migration of dermal tissue or cutaneous ectoderm from the caudal margin of the structures, where skin edges were attached.[
Rostral margins of the NP might be where dermal elements tend to be left behind at the initial surgery, as shown in Case 3. Because the dermal layer can grow over a pia-arachnoid membrane of the intermediate zone and sometimes a NP,[
An epidermoid cyst occurred in delayed fashion in Case 1, in which the capsule did not exist in the vicinity of the previous border to the TF-LS presumed from the location of the knots used at the first surgery, but were located more rostrally. This finding suggests that the epidermoid cyst might not have developed from the dermoid element that was continuous with that in the TF-LS. In that case, one possible origin is a remnant of dermal element on the rostral margin of the NP at the first surgery, as shown in Case 3. A second possible origin is another congenital dermoid element in the NP that had existed independently of the one in the TF-LS, because dermoid elements are speculated to exist anywhere in MMC site at birth due to common embryological origin for MMC and the dermoid elements during neural tube formation.[
The present findings suggest that dermoid/epidermoid cysts occurring later could be derived from both prenatally existing cryptic dermoid elements in neural structures and remnants of dermal elements overlapping the margins of NP. Total removal of dermal elements on or in functional neural structures might be sometimes difficult. It has been reported that the delayed occurrence of dermoid cyst was observed in patients who underwent prenatal MMC repair surgery with no significant difference or a slightly increased rate compared to those underwent postnatal repair.[
The incidence of dermoid/epidermoid cysts after repair surgery for MMC has been reported to be 9–16%.[
CONCLUSION
Prenatally existing cryptic dermoid elements in the caudal portion of neural structures and remnants of dermal elements overlapping the rostral margin of the NP are associated with delayed occurrence of dermoid/epidermoid cysts. Postoperative histopathological investigation of the resected specimens is recommended. Once dermal elements are revealed, repeated MRI examination including 3D hT2WI and additional surgery, when required, should be considered.
Ethics statement
The authors confirm that written informed consent was obtained from the families of the infants described in this report.
The authors declare that this work complies with the guidelines for human studies, and the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Research Foundation of Fukuoka Children’ Hospital.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
References
1. Adzick NS, Thom EA, Spong CY, Brock JW, Burrows PK, Johnson MP. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011. 364: 993-1004
2. Chadduck WM, Roloson GJ. Dermoid in the filum terminale of a newborn with myelomeningocele. Pediatr Neurosurg. 1993. 19: 81-3
3. Danzer E, Adzick NS, Rintoul NE, Zarnow DM, Schwartz ES, Melchionni J. Intradural inclusion cysts following in utero closure of myelomeningocele: Clinical implications and follow-up findings. J Neurosurg Pediatr. 2008. 2: 406-13
4. Dewan MC, Wellons JC. Fetal surgery for spina bifida. J Neurosurg Pediatr. 2019. 24: 105-14
5. Fiaschi P, Piatelli G, Cama A, Capra V, Consales A, Ravegnani M. Intraspinal inclusion tumor after myelomeningocele repair: A long-term single-center experience. World Neurosurg. 2019. 122: e1338-44
6. Gupta N. Surgical techniques for open fetal repair of myelomeningocele. Childs Nerv Syst. 2017. 33: 1143-8
7. Hashiguchi K, Morioka T, Yoshida F, Miyagi Y, Mihara F, Yoshiura T. Feasibility and limitation of constructive interference in steady-state (CISS) MR imaging in neonates with lumbosacral myeloschisis. Neuroradiology. 2007. 49: 579-85
8. Hashiguchi K, Morioka T, Murakami N, Togao O, Hiwatashi A, Ochiai M. Clinical significance of prenatal and postnatal heavily T2-weighted magnetic resonance images in patients with myelomeningocele. Pediat Neurosurg. 2015. 50: 310-20
9. Hutchins GM, Meuli M, Meuli-Simmen C, Jordan MA, Heffez DS, Blakemore KJ. Acquired spinal cord injury in human fetuses with myelomeningocele. Pediatr Pathol Lab Med. 1996. 16: 701-12
10. Kurogi A, Morioka T, Murakami N, Nakanami N, Suzuki SO. Ruptured dermoid cyst of the conus medullaris in the myelomeningocele sac revealed at the initial repair surgery. Childs Nerv Syst. 2020. 36: 1061-5
11. Martinez-Lage JF, Poza M, Sola J. Dermoid and epidermoid tumors in myelomeningocele patients. Pediatr Neurosurg. 1994. 20: 274
12. Morioka T, Hashiguchi K, Mukae N, Sayama T, Sasaki T. Neurosurgical management of patients with lumbosacral myeloschisis. Neurol Med Chir (Tokyo). 2010. 50: 870-6
13. Morioka T, Murakami N, Kanata A, Tsukamoto H, Suzuki SO. Retained medullary cord with sacral subcutaneous meningocele and congenital dermal sinus. Childs Nerv Syst. 2020a. 36: 423-7
14. Morioka T, Murakami N, Ichiyama M, Kusuda T, Suzuki SO. Congenital dermal sinus elements in each tethering stalk of coexisting thoracic limited dorsal myeloschisis and retained medullary cord. Pediatr Neurosurg. 2020b. 55: 380-7
15. Murakami N, Morioka T, Shimogawa T, Hashiguchi K, Mukae N, Uchihashi K. Retained medullary cord extending to a sacral subcutaneous meningocele. Childs Nerv Syst. 2018. 34: 527-33
16. Murakami N, Kanata A, Kurogi A, Mukae N, Shimogawa T, Nakanami N. Myelomeningocele in one neonate from a fraternal triplet birth: Two case reports on neurosurgical and multidisciplinary treatment during the perinatal period. Interdiscip Neurosurg. 2022. 27: 101372
17. Pang D, Zovickian J, Moes GS. Retained medullary cord in humans: Late arrest of secondary neurulation. Neurosurgery. 2011. 68: 1500-19 discussion 1519
18. Pugh JA, Aronyk KE, Norton JA. Neural activity generated in the neural placode and nerve roots in the neonate with spina bifida. J Neurosurg Pediatr. 2012. 9: 452-6
19. Ramos E, Marlin AE, Gaskill SJ. Congenital dermoid tumor in a child at initial myelomeningocele closure: An etiological discussion. J Neurosurg Pediatr. 2008. 2: 414-5
20. Schindelmann KH, Paschereit F, Steege A, StoltenburgDidinger G, Kaindl AM. Systematic classification of spina bifida. J Neuropathol Exp Neurol. 2021. 80: 294-305
21. Scott RM, Wolpert SM, Bartoshesky LE, Zimbler S, Klauber GT. Dermoid tumors occurring at the site of previous myelomeningocele repair. J Neurosurg. 1986. 65: 779-83
22. Sival DA, van Weerden TW, Vles JS, Timmer A, den Dunnen WF, Staal-Schreinemachers AL. Neonatal loss of motor function in human spina bifida aperta. Pediatrics. 2004. 114: 427-34
23. Storrs BB. Are dermoid and epidermoid tumors preventable complications of myelomeningocele repair?. Pediatr Neurosurg. 1994. 20: 160-2
24. Van Aalst J, Hoekstra F, Beuls EA, Cornips EM, Weber JW, Sival DA. Intraspinal dermoid and epidermoid tumors: Report of 18 cases and reappraisal of the literature. Pediatr Neurosurg. 2009. 45: 281-90