- Department of Neurosurgery, Neurological Institution of Thailand, Bangkok, Thailand
- Department of Neuroradiology, Neurological Institution of Thailand, Bangkok, Thailand
- Department of Radiology, Bumrungrad International Hospital, Bangkok, Thailand.
Correspondence Address:
Prasert Iampreechakul, Department of Neurosurgery, Neurological Institution of Thailand, Bangkok, Thailand.
DOI:10.25259/SNI_138_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Prasert Iampreechakul1, Intouch Sopchokchai1, Korrapakc Wangtanaphat1, Songpol Chuntaroj2, Yodkhwan Wattanasen2, Sunisa Hangsapruek2, Punjama Lertbutsayanukul2, Somkiet Siriwimonmas3. Holocord myelopathy misdiagnosed as neuromyelitis optica spectrum disorder (NMOSD): A unique case of dural arteriovenous fistula at the craniocervical junction along first spinal nerve. 21-Jun-2024;15:209
How to cite this URL: Prasert Iampreechakul1, Intouch Sopchokchai1, Korrapakc Wangtanaphat1, Songpol Chuntaroj2, Yodkhwan Wattanasen2, Sunisa Hangsapruek2, Punjama Lertbutsayanukul2, Somkiet Siriwimonmas3. Holocord myelopathy misdiagnosed as neuromyelitis optica spectrum disorder (NMOSD): A unique case of dural arteriovenous fistula at the craniocervical junction along first spinal nerve. 21-Jun-2024;15:209. Available from: https://surgicalneurologyint.com/surgicalint-articles/12953/
Abstract
Background: Dural arteriovenous fistulas (DAVFs) at the craniocervical junction (CCJ) involving the first spinal nerve represent a particularly rare and challenging subtype of DAVFs, with holocord myelopathy secondary to cerebrospinal DAVFs being an exceedingly rare presentation.
Case Description: We report the case of a 70-year-old woman who presented with progressive paraparesis over 2 weeks. Initial magnetic resonance imaging (MRI) of the spine showed extensive holocord myelopathy, leading to a misdiagnosis of inflammatory myelopathy and subsequent inappropriate steroid treatment at a local hospital, which exacerbated her neurological symptoms. On transfer to our institution and further evaluation with MRI and magnetic resonance angiography, a lower thoracic DAVF was initially suspected. However, comprehensive spinal angiography failed to localize the fistula, prompting cranial angiography, which ultimately identified a DAVF at the CCJ along the C1 nerve root, supplied by a small radiculomeningeal branch of the left vertebral artery. Successful management involved coagulation of the proximal draining vein, with follow-up imaging confirming complete fistula obliteration and resolution of the holocord edema.
Conclusion: This case highlights the diagnostic and therapeutic challenges associated with DAVFs at the CCJ, particularly when presenting with holocord myelopathy. It underscores the importance of a high index of suspicion and the need for timely, accurate diagnosis and intervention to prevent permanent spinal cord damage in such rare and complex cases.
Keywords: Craniocervical junction, Dural arteriovenous fistula, First cervical spinal nerve, Holocord myelopathy, Longitudinally extensive transverse myelitis, Neuromyelitis optica spectrum disorder
INTRODUCTION
Intracranial dural arteriovenous fistulas (DAVFs) are rare and complex vascular malformations characterized by abnormal connections between meningeal arteries and dural venous sinuses or cortical veins.[
Despite advancements in neuroimaging and therapeutic techniques, DAVFs located at the CCJ continue to present significant challenges due to the complexity of vascular anatomy in this region, the variability of clinical presentations, and the potential for considerable morbidity and mortality. It is now recognized that arteriovenous fistula (AVFs) of the CCJ should be specifically referred to when they are located at the C1 or C2 levels. High cervical CCJ AVFs can be categorized into four types: DAVF, radicular AVF, epidural AVF, and perimedullary AVF.[
Holocord myelopathy, characterized by edema encompassing the entire spinal cord, is a rare but severe clinical entity that can result in significant morbidity if not promptly identified and managed. The occurrence of this condition due to AVFs at intracranial or CCJ locations is exceedingly rare.[
CASE DESCRIPTION
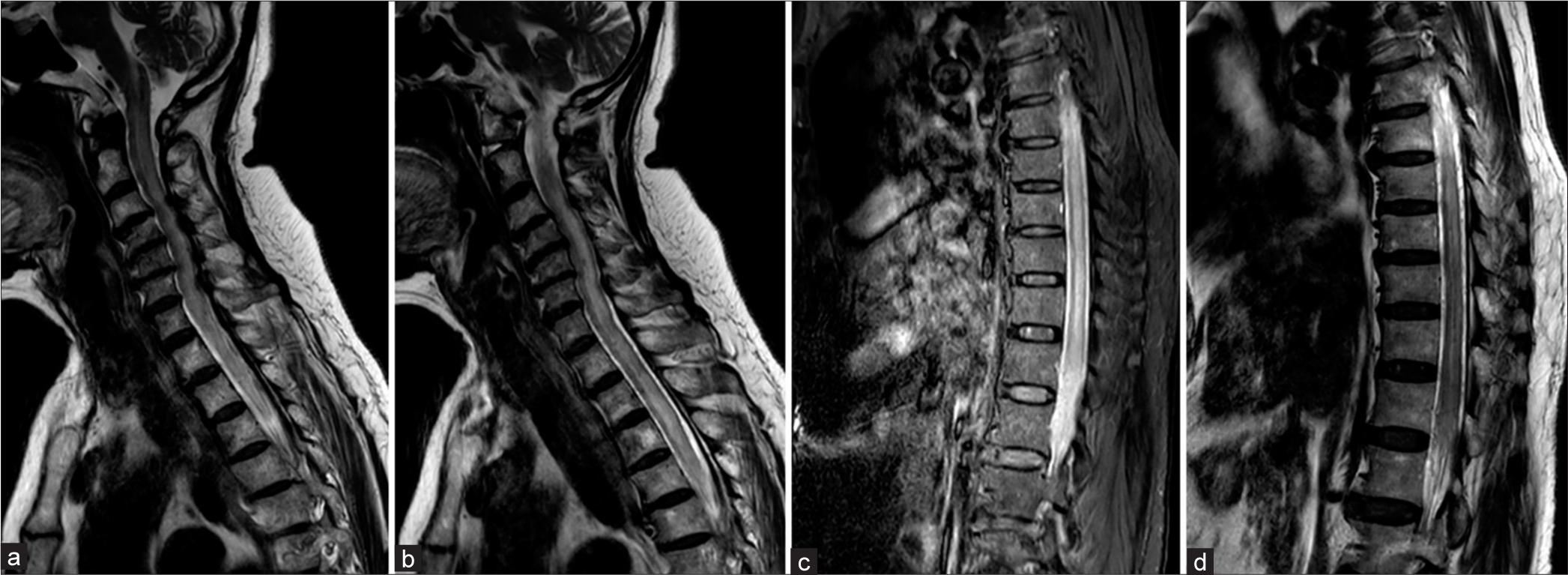
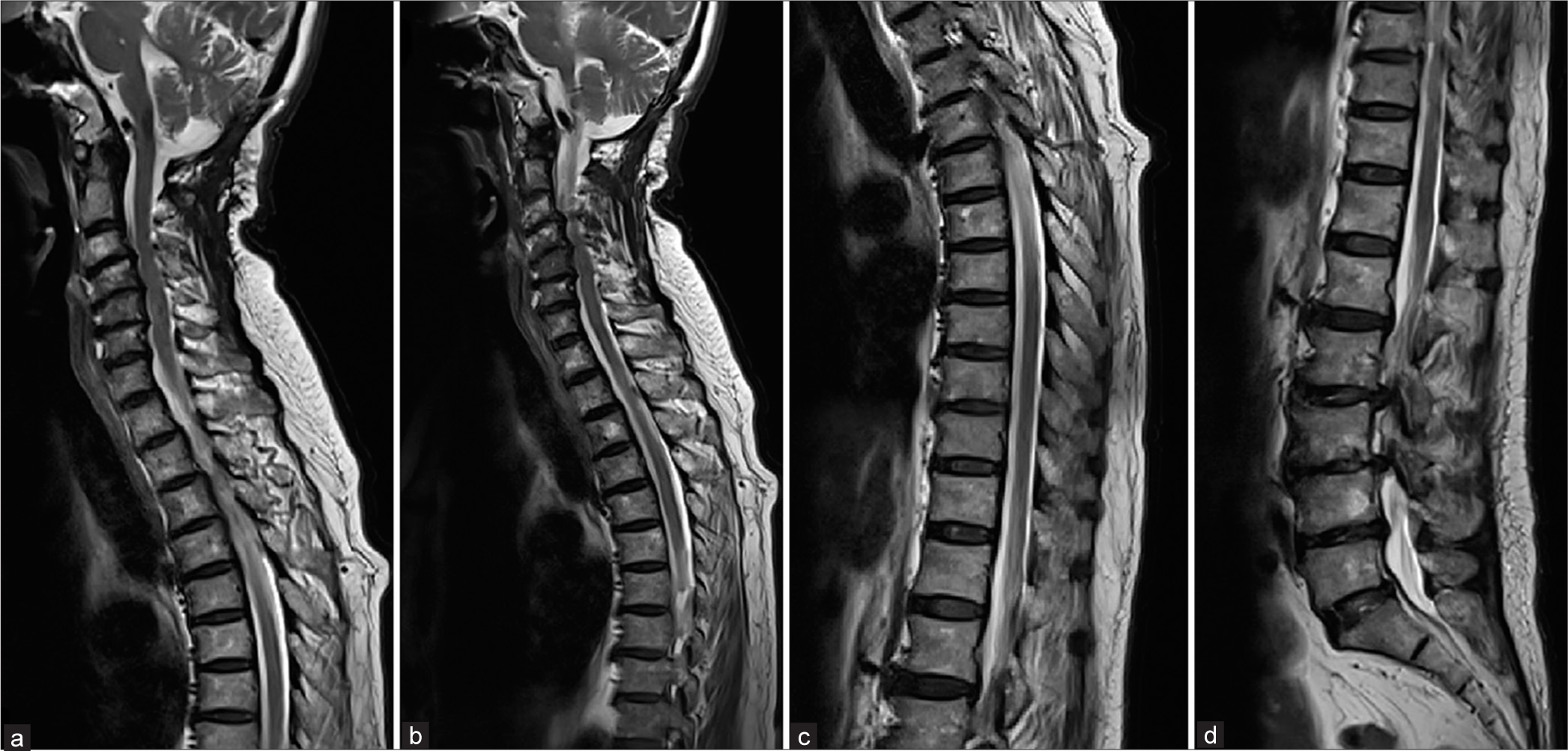
A 70-year-old woman with a history of hypertension, dyslipidemia, and chronic neck pain for 3 years was admitted to a local hospital after experiencing progressive paraparesis over 2 weeks. She reported accompanying neck and back pain but no visual or bulbar symptoms nor a history of trauma. Two days before her hospital admission, she developed bowel and bladder dysfunction. Magnetic resonance imaging (MRI) with a 1.5 Tesla scanner of the entire spine revealed an abnormal T2 hyperintensity affecting both the gray and white matter, sparing only the peripheral regions of the spinal cord, extending from the cervicomedullary junction to the conus medullaris, indicative of holocord edema. In addition, disc herniation and thickening of the ligamentum flavum were observed, causing moderate to severe spinal stenosis at the levels of C3–4, C4–5, C5–6, and C6–7. No abnormal flow voids were reported in the radiological analysis [
Figure 1:
1.5 Tesla Magnetic resonance imaging of the whole spine obtained 2 weeks after initial symptom of progressive paraparesis. (a-d) Sagittal T2-weighted images of cervical, thoracic, and lumbar spine show abnormal hyperintensity, representing spinal cord edema of the whole spinal cord. There are disc herniation and spinal stenosis at the level of C3-4, C4-5, C5-6, and C6-7.
Figure 2:
1.5 Tesla Magnetic resonance imaging of spine obtained 2 weeks after initial symptom of progressive paraparesis. (a) Sagittal contrast-enhanced T1-weighted image of cervicothoracic spine shows patchy enhancement of spinal cord. (b, c) Axial T2-weighted images of T6 and T9 levels reveal spinal cord edema with sparing of peripheral white matter.
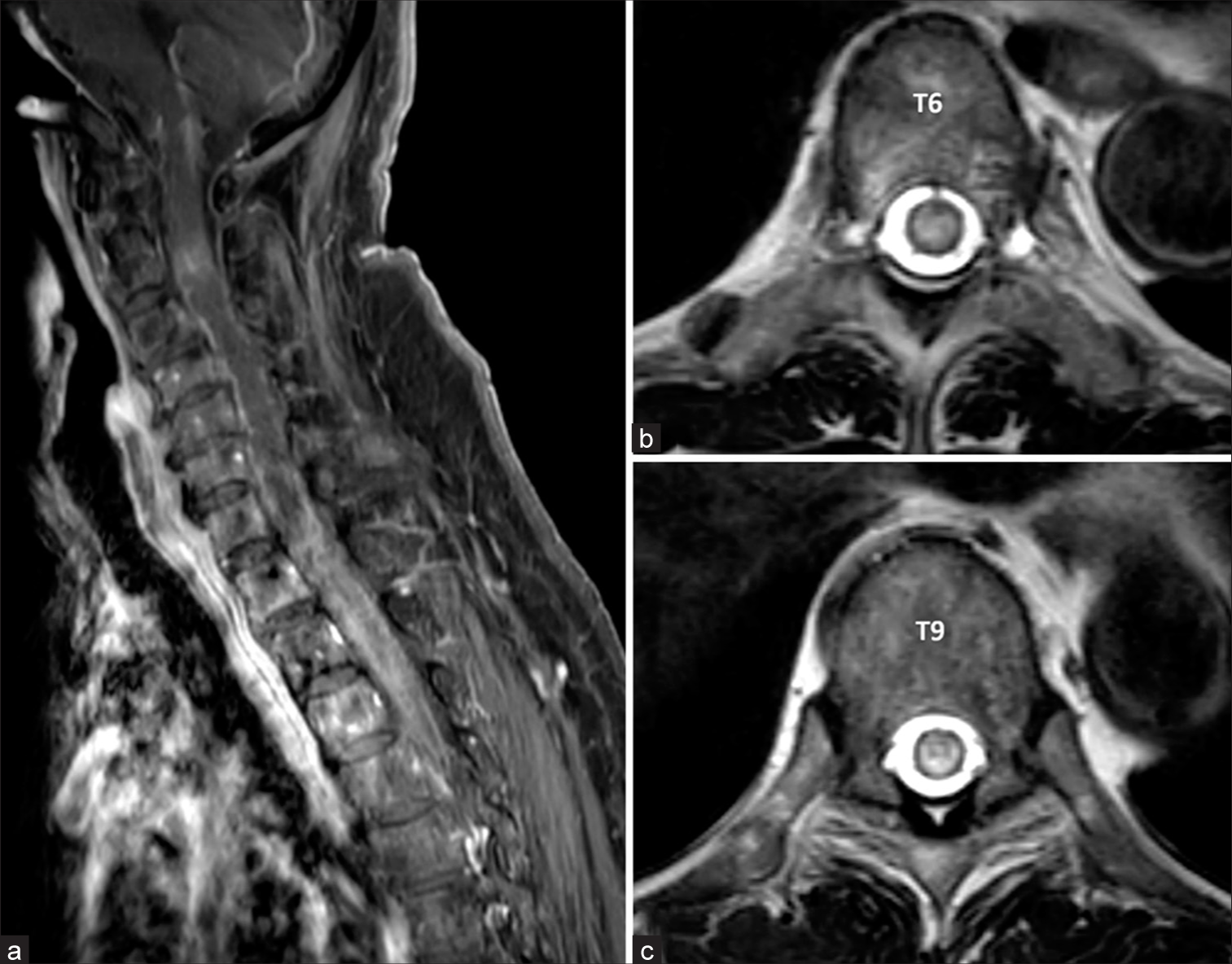
On neurological examination at our institute, evidence of spastic paraparesis was noted, with muscle strength rated at 1–2/5. In addition, the patient lacked pinprick sensation below the T6 level and showed impaired proprioception below the knees. A follow-up MRI using a 3 Tesla scanner and 3D T2-weighted sampling perfection with application-optimized contrasts using different flip angle evolution (SPACE) conducted 1 month after the initial symptoms emerged showed the spinal cord edema extending up to the lower brainstem, with subtle perimedullary flow voids at the thoracic level. The imaging also confirmed severe spinal stenosis at L2–3 and L3–4 levels due to disc herniation and ligamentum flavum thickening [
Figure 3:
3 Tesla Magnetic resonance imaging of the whole spine obtained 1 month later. (a, b) Sagittal and (c, d) coronal 3D T2-SPACE images of cervicothoracic and thoracolumbar spine reveal the progression of venous congestion extending from lower brainstem to conus medullaris with subtle perimedullary flow voids (denoted by arrowheads in figure 3a). There are disc herniation and severe spinal stenosis at the level of C3-4, L3-4, and L4-5. SPACE: Sampling perfection with application-optimized contrasts using different flip angle evolution.
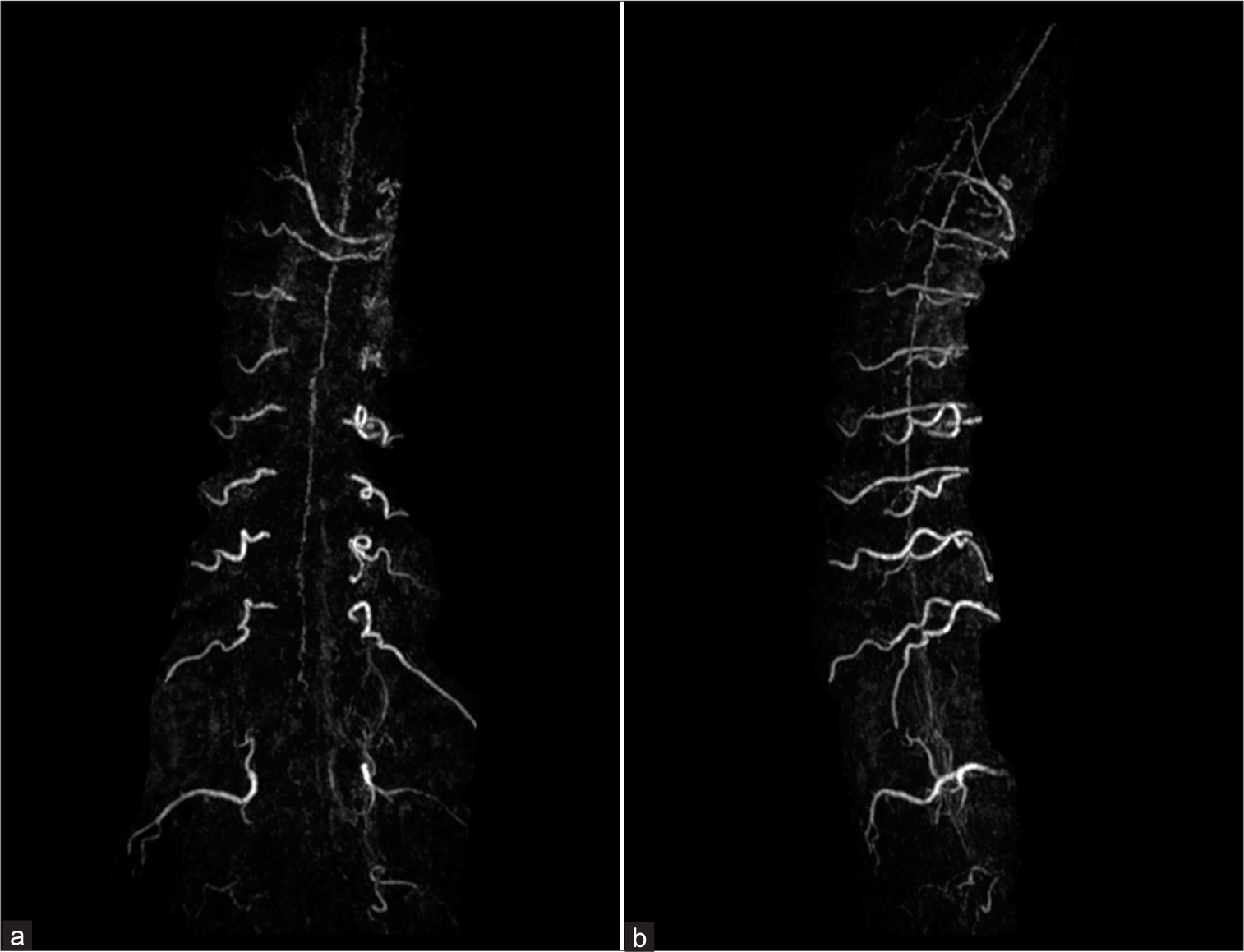
Figure 4:
(a) Coronal and (b) sagittal maximum intensity projection images from contrast-enhanced magnetic resonance angiography of the whole spine demonstrate dilated and tortuous veins mainly along the anterior surface of the spinal cord extending from the conus medullaris to the cervical level, suspecting left-sided spinal dural arteriovenous fistula at lower thoracic level.
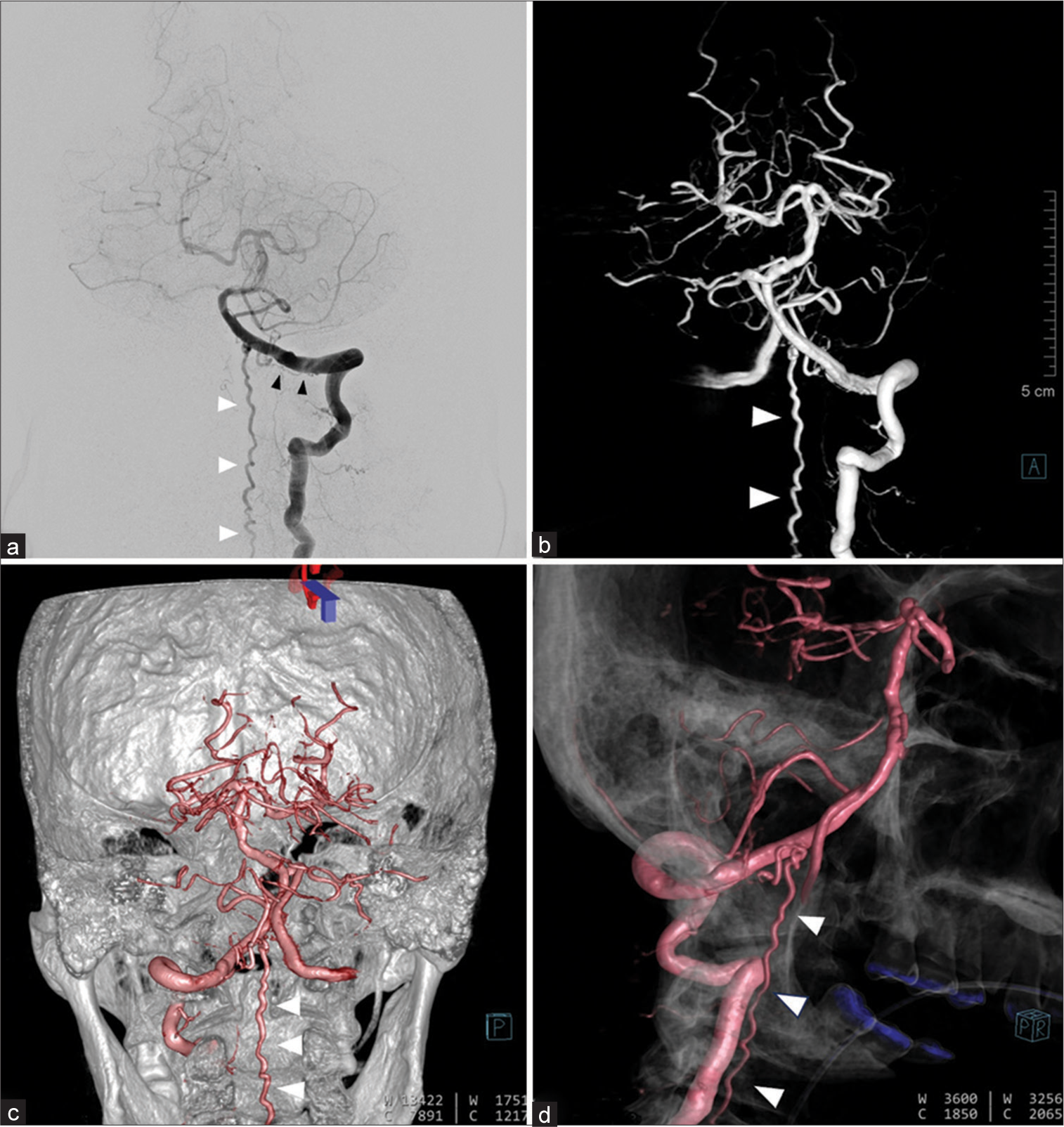
Figure 5:
(a) Anteroposterior view of the left vertebral artery (VA) injection reveals the left dural arteriovenous fistula of craniocervical junction supplied by the radiculomeningeal branch (black arrowheads) of left VA from C1 level with draining veins extending rostrally to the medulla, and caudally to the cervical cord (white arrowheads) through dilated left lateral medullary vein. (b) Anteroposterior, (c) posteroanterior, and (d) lateral views of 3D reconstruction images of the left VA clearly demonstrate an enlarged and tortuous anterior spinal vein (white arrowheads).
Given the challenging nature of the fistula’s small and tortuous feeding artery, surgical intervention was deemed necessary. The patient underwent a left suboccipital craniotomy and C1 hemilaminectomy in a right lateral decubitus position. The vertebral artery and proximal draining vein were identified, and the proximal draining vein’s disconnection was confirmed with intraoperative indocyanine green angiography, which showed no fluorescent flow post-coagulation [
Figure 6:
(a) Intraoperative photograph during surgery on the right lateral decubitus position after opening the dura. The proximal draining vein (green asterisk) is identified after the dissection of the first denticulate ligament. (b) After coagulation and disconnection of the draining vein, intraoperative indocyanine green angiography confirms the absence of fluorescent flow through the draining vein.
Figure 7:
(a) Anteroposterior view of the left vertebral artery injection obtained 2 weeks after surgery confirms complete obliteration of the fistula. (b) Coronal maximum intensity projection image from contrast-enhanced magnetic resonance angiography of the vertebrobasilar system obtained 4 months after operation also reveals no residual fistula.
DISCUSSION
Our patient was initially misdiagnosed radiologically as having LETM, a condition characterized by a hyperintense intramedullary signal on spinal MRI that extends beyond three vertebral segments. NMOSD, which predominantly affects females, is a leading cause of LETM. Other causes of LETM include multiple sclerosis, myelin oligodendrocyte glycoprotein antibody disorders, acute disseminated encephalomyelitis, spinal cord infarction, parainfectious myelopathy, paraneoplastic myelitis, and spinal DAVF.[
The acute clinical deterioration following methylprednisolone pulse therapy in our patient highlights a crucial diagnostic consideration. Such worsening after steroid administration may suggest the presence of a spinal DAVF, warranting further imaging to confirm the diagnosis. This is especially relevant in the setting of spinal DAVFs located in the upper cervical spine or Cognard type V fistulas, intracranial DAVFs draining into peri medullary veins, in which progression of myelopathy could result in acute tetraparesis or respiratory failure.[
Utilizing 3T MRI with 3D T2-weighted SPACE allowed for the detection of perimedullary flow voids in our patient, raising suspicion for a spinal DAVF. This imaging technique offers high spatial resolution without the need for contrast, improving the sensitivity in detecting spinal DAVFs and aiding in differential diagnosis from conditions like acute transverse myelitis.[
DAVFs of the CCJ along the C1 spinal nerve are a rare and distinct subtype. These fistulas create an abnormal direct connection between the arterial and venous systems, bypassing the capillary network and resulting in blood flowing directly from high-pressure arterial vessels into lower-pressure venous channels, leading to venous hypertension or congestion.[
Interestingly, holocord edema occurred in our patient with C1 spinal nerve DAVF, probably low-flow shunt due to a single small feeder from the radiculomeningeal branch of VA. The mechanism of holocord edema remains unclear. Due to probably a long-standing lesion in our patient, the sustained high venous pressure may disrupt the blood-spinal cord barrier, and subsequent edema can trigger an inflammatory response within the spinal cord, resulting in further damaging of neural tissue and exacerbating edema that worsens the extent of myelopathy. The draining veins may extend downward as far as a lumbosacral region to reach the outlet.[
Most high cervical AVFs can be effectively treated with open surgery. Endovascular treatment remains challenging due to a high rate of incomplete obliteration and complications, and it can only be performed in superselective AVFs with simple angioarchitecture. Appropriate treatment can lead to a good prognosis.[
CONCLUSION
DAVF at the CCJ involving the first spinal nerve, coupled with holocord myelopathy, represents an exceedingly rare clinical entity with a propensity for rapid progression and severe outcomes. This case underscores the critical importance of early and accurate diagnosis, alongside prompt therapeutic intervention, to mitigate the risk of irreversible spinal cord damage. The complicating factor of spinal canal stenosis, by altering CSF dynamics, may further aggravate the condition, acting in concert with the longstanding venous hypertension induced by a C1 nerve root DAVF. This synergistic interaction underscores a complex pathophysiological mechanism, highlighting the need for a comprehensive diagnostic approach to identify and effectively manage such challenging cases, thereby preventing the devastating consequences of untreated spinal DAVFs.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Chandrasekar S, John J, Satapathy AK. Longitudinally extensive transverse myelitis: One disease, variable outcomes-a case series. J Neurosci Rural Pract. 2022. 13: 339-42
2. Hayashida S, Masaki K, Matsushita T, Watanabe M, Yamasaki R, Murai H. Holocord involvement with sparing of the peripheral white matter rim in longitudinally extensive spinal cord lesions of neuromyelitis optica. Clin Exp Neuroimmunol. 2015. 6: 78-9
3. Iampreechakul P, Polpong P, Wangtanaphat K, Lertbutsayanukul P, Wattanasen Y, Siriwimonmas S. Acquired lumbosacral spinal Dural arteriovenous fistula in association with degenerative lumbosacral disc herniation and spinal canal stenosis: Report of two cases and review of the literature. Asian J Neurosurg. 2020. 15: 1059-67
4. Iampreechakul P, Wangtanaphat K, Chuntaroj S, Wattanasen Y, Hangsapruek S, Lertbutsayanukul P. Spontaneous complete regression of malignant cavernous sinus Dural arteriovenous fistula following partial transarterial embolization with liquid embolic material: Report of two cases. Surg Neurol Int. 2023. 14: 307
5. Iampreechakul P, Wangtanaphat K, Hangsapruek S, Wattanasen Y, Lertbutsayanukul P, Siriwimonmas S. Acquired Chiari malformation Type I and holocord syringomyelia associated with a high-flow supratentorial fistulous arteriovenous malformations: A case report and literature review. Surg Neurol Int. 2022. 13: 217
6. Iampreechakul P, Wangtanaphat K, Lertbutsayanukul P, Wattanasen Y, Siriwimonmas S. Spontaneous closure of a cavernous sinus Dural arteriovenous fistula with spinal perimedullary drainage (Cognard V) during attempted transvenous embolization. Asian J Neurosurg. 2019. 14: 1268-74
7. Iampreechakul P, Wangtanaphat K, Wattanasen Y, Hangsapruek S, Lertbutsayanukul P, Siriwimonmas S. Dural arteriovenous fistula of the craniocervical junction along the first cervical nerve: A single-center experience and review of the literature. Clin Neurol Neurosurg. 2023. 224: 107548
8. Kim CH, Chung CK, Kwon BJ, Kim HJ. Holocord myelopathy with thoracic stenosis: Case report and hypothesis. Spinal Cord. 2003. 41: 696-9
9. Lee JY, Cho YD, Kwon BJ, Han MH. Dural arteriovenous fistula at the foramen magnum with holocord myelopathy: Case report. Neurointervention. 2010. 5: 53-7
10. Li J, Lin F, Zhu J, Zhuo L, Chen F, Dai L. Enhanced treatment options for Dural arteriovenous fistulas at the craniocervical junction: Endovascular embolization versus microsurgery? A single-center 23-year experience. World Neurosurg. 2024. 182: e414-30
11. Nasr DM, Brinjikji W, Rabinstein AA, Lanzino G. Clinical outcomes following corticosteroid administration in patients with delayed diagnosis of spinal arteriovenous fistulas. J Neurointerv Surg. 2017. 9: 607-10
12. Ouyang F, Wu Q, Chen Y, Yin M, Liu J, Lv L. The value of 3D T2-weighted SPACE sequence in the differential diagnosis of spinal arteriovenous fistula and acute transverse myelitis. Eur Spine J. 2023. 32: 4111-7
13. Paudel S, Nepal G, Guragain S, Shah S, Paudel BS, Ojha R. Longitudinally extensive transverse myelitis: A retrospective study differentiating neuromyelitis optica spectrum disorder from other etiologies. Cureus. 2021. 13: e13968
14. Punia P, Chugh A, Gotecha S, Lachake A. Single-level ossified ligamentum flavum causing a holocord syrinx: Illustrative case. J Neurosurg Case Lessons. 2023. 6: CASE23340
15. Su H, Yu J. Treatment of high cervical arteriovenous fistulas in the craniocervical junction region. Front Neurol. 2023. 14: 1164548
16. Tsurubuchi T, Matsumura A, Nakai K, Fujita K, Enomoto T, Iwasaki N. Reversible holocord edema associated with intramedullary spinal abscess secondary to an infected dermoid cyst. Pediatr Neurosurg. 2002. 37: 282-6
17. Wada K, Tanei T, Hattori K, Hatano H, Fujitani S, Ito R. Unique vascular structures of a radicular arteriovenous fistula at the craniocervical junction along the first cervical spinal nerve: A case report. Surg Neurol Int. 2023. 14: 85
18. Whittam D, Huda S, Gibbons E, Pullicino R, Solomon T, Chandran A. A case series of intracranial Dural arteriovenous fistulae mimicking cervical myelitis: A diagnosis not to be missed. J Neurol. 2021. 268: 4680-6
19. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015. 85: 177-89