- Department of Neurosurgery, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Ortopedico Galeazzi, Milan, Italy,
- Pathology Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) San Raffaele Scientific Institute, Milan, Italy,
- University of Basel, Basel, Switzerland,
- Department of Spine Surgery, Swiss Paraplegic Center, Nottwil, Switzerland.
- Department of Anesthesia, Surgical Intensive Care, Preclinical Emergency Medicine and Pain Therapy, University Hospital Basel, Basel, Switzerland.
Correspondence Address:
Edvin Zekaj, Department of Neurosurgery, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Ortopedico Galeazzi, Milan, Italy.
DOI:10.25259/SNI_620_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Edvin Zekaj1, Marcella Callea2, Christian Saleh3, Guglielmo Iess1, Phillip Jaszczuk4, Luzius A. Steiner5, Viktorija Kenstaviciute5, Domenico Servello1. How to avoid intraoperative complications of active paragangliomas?. 24-Nov-2023;14:405
How to cite this URL: Edvin Zekaj1, Marcella Callea2, Christian Saleh3, Guglielmo Iess1, Phillip Jaszczuk4, Luzius A. Steiner5, Viktorija Kenstaviciute5, Domenico Servello1. How to avoid intraoperative complications of active paragangliomas?. 24-Nov-2023;14:405. Available from: https://surgicalneurologyint.com/surgicalint-articles/12646/
Abstract
Background: Paragangliomas (PGs) are very rare neuroendocrine tumors that can be found in unusual locations such as the spinal canal. Some PGs may be endocrinologically active, containing neurotransmitters such as noradrenaline, adrenaline, and serotonin. This can lead to unexpected neurotransmitter release during the removal of PGs, leading to a hypertensive crisis.
Case Description: We present two patients who underwent surgical removal of a secretory filum terminale PG.
Conclusion: If laboratory tests are suggestive of a secretory tumor, surgery should include anesthesiologic preparation similar to cases of pheochromocytoma.
Keywords: Neuroendocrine tumors, Paraganglioma, Surgery, Complications, Pre-operative Screening
INTRODUCTION
Paragangliomas (PGs) are rare neuroendocrine tumors mostly found in the carotid body, thoracoabdominal sympathetic nerves, and glomus jugulare. Still, they may also occur in other unusual sites, such as the spinal canal.[
CASE 1
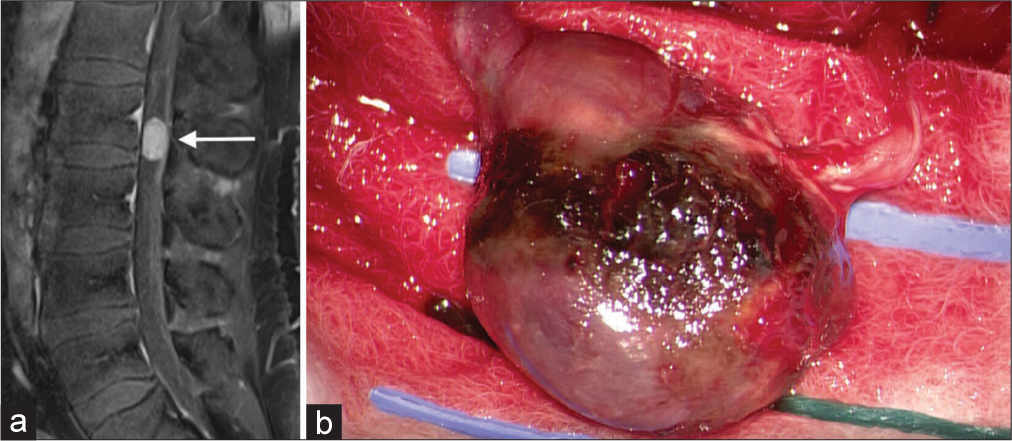
A 48-year-old male patient presented with back pain lasting two years, radiating into both lower extremities. Associated symptoms were severe episodes of anxiety and tachycardia, to which, initially, not sufficient attention was given. Lumbar spine MRI showed a contrast-enhancing intradural lesion occupying the spinal canal at L2 on T1-weighted MRI [
CASE 2
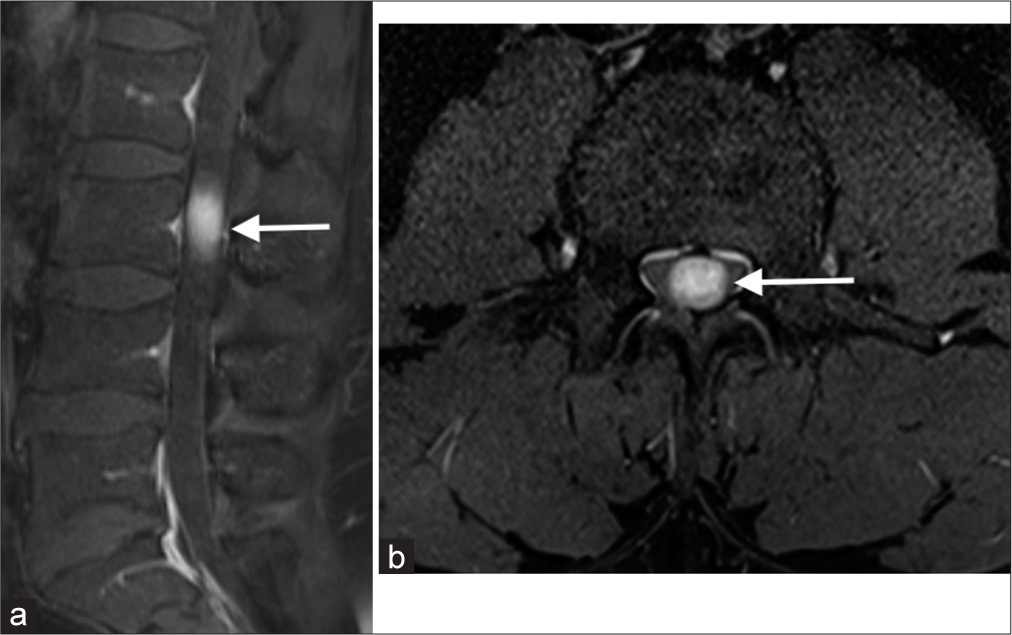
A 48-year-old male patient presented to our department for chronic low back pain. The patient had also been referred separately for evaluation of panic attacks and an anxiety disorder in the previous two years. A lumbosacral MRI showed an intradural lesion behind the L3 vertebral body, which showed homogeneous enhancement [
HISTOPATHOLOGICAL ANALYSIS
Patient 1
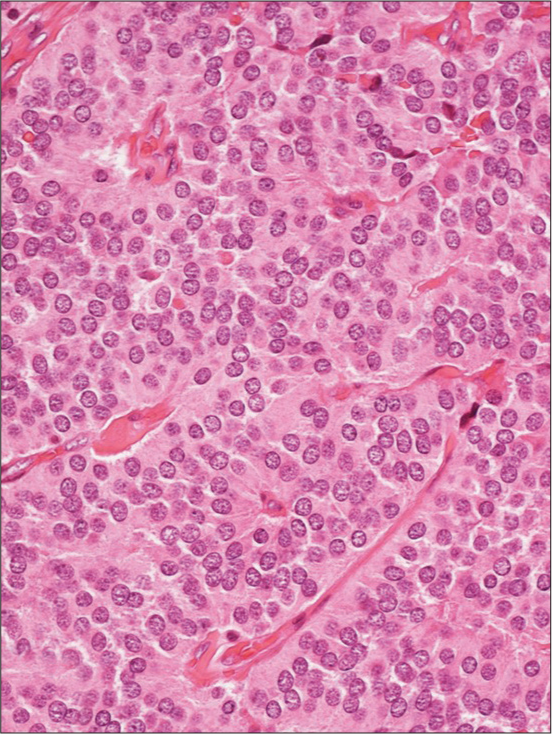
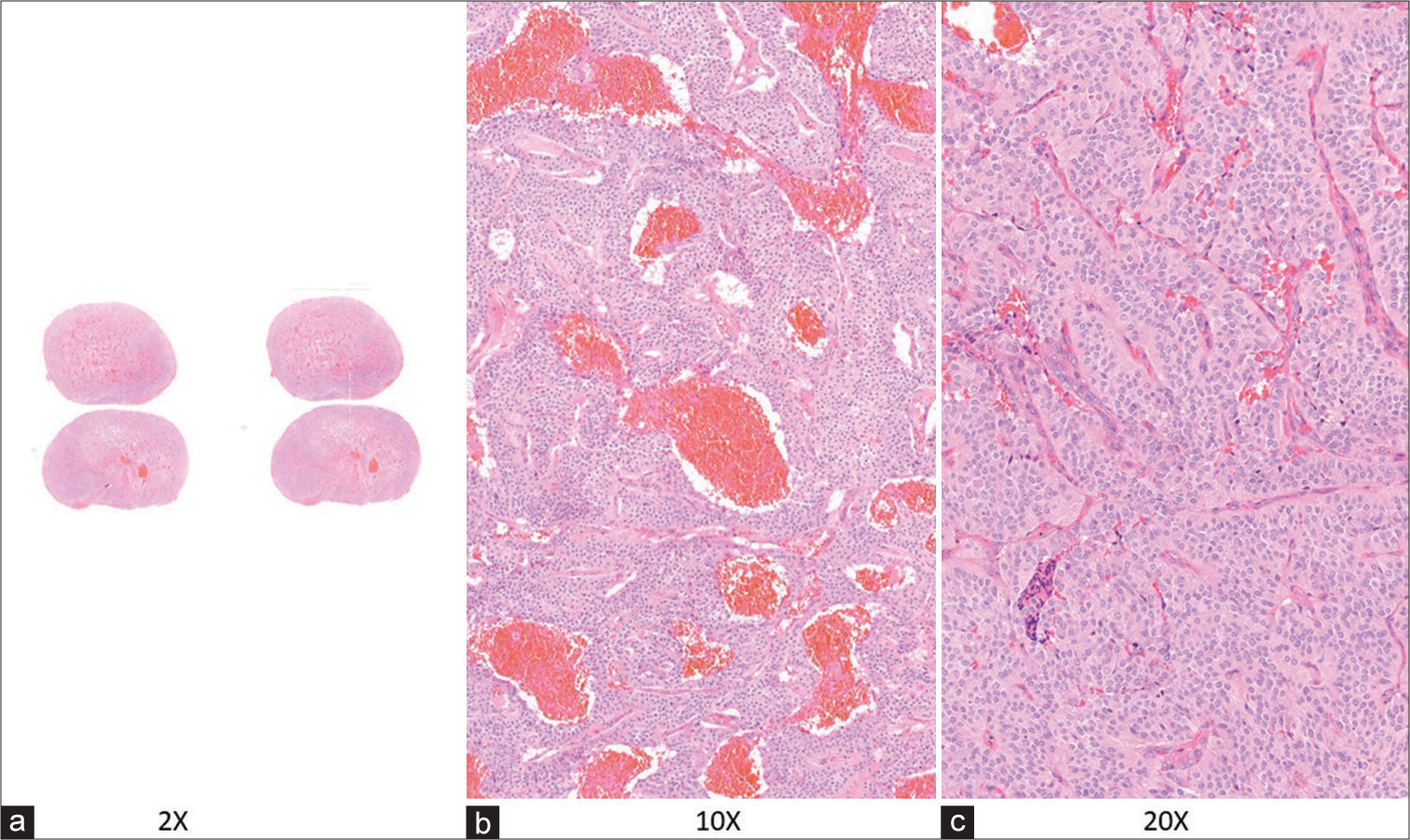
The specimen was a two × 1.2 × 1 cm encapsulated brown nodule with a homogeneous appearance. Microscopically, the lesion had sharp borders, and it was surrounded by fibrous tissue with focal intra- and peri-capsular calcifications. The nodule consisted of a benign neoplastic proliferation of cells with round/oval nuclei and finely dispersed chromatin; tumor cells were arranged in lobules and nests with an extensive intervening capillary network [
Then, a few vessels showed hyaline walls, and others were congested; necrosis, vascular, and capsular invasion were not identified.
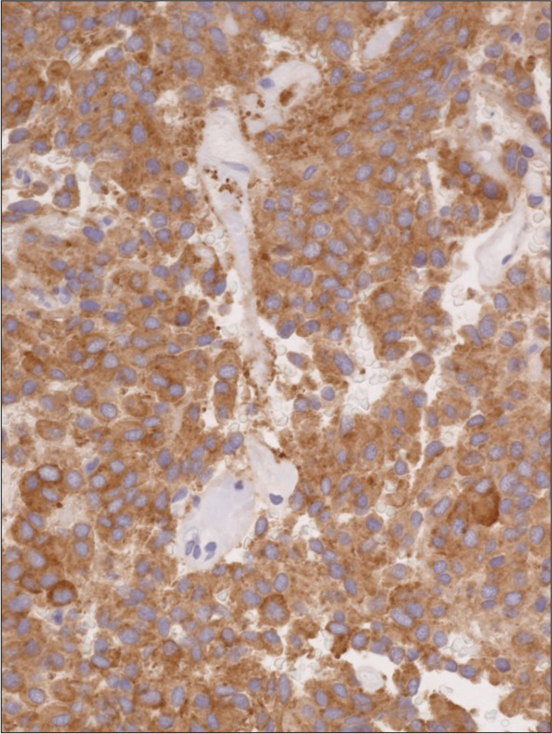
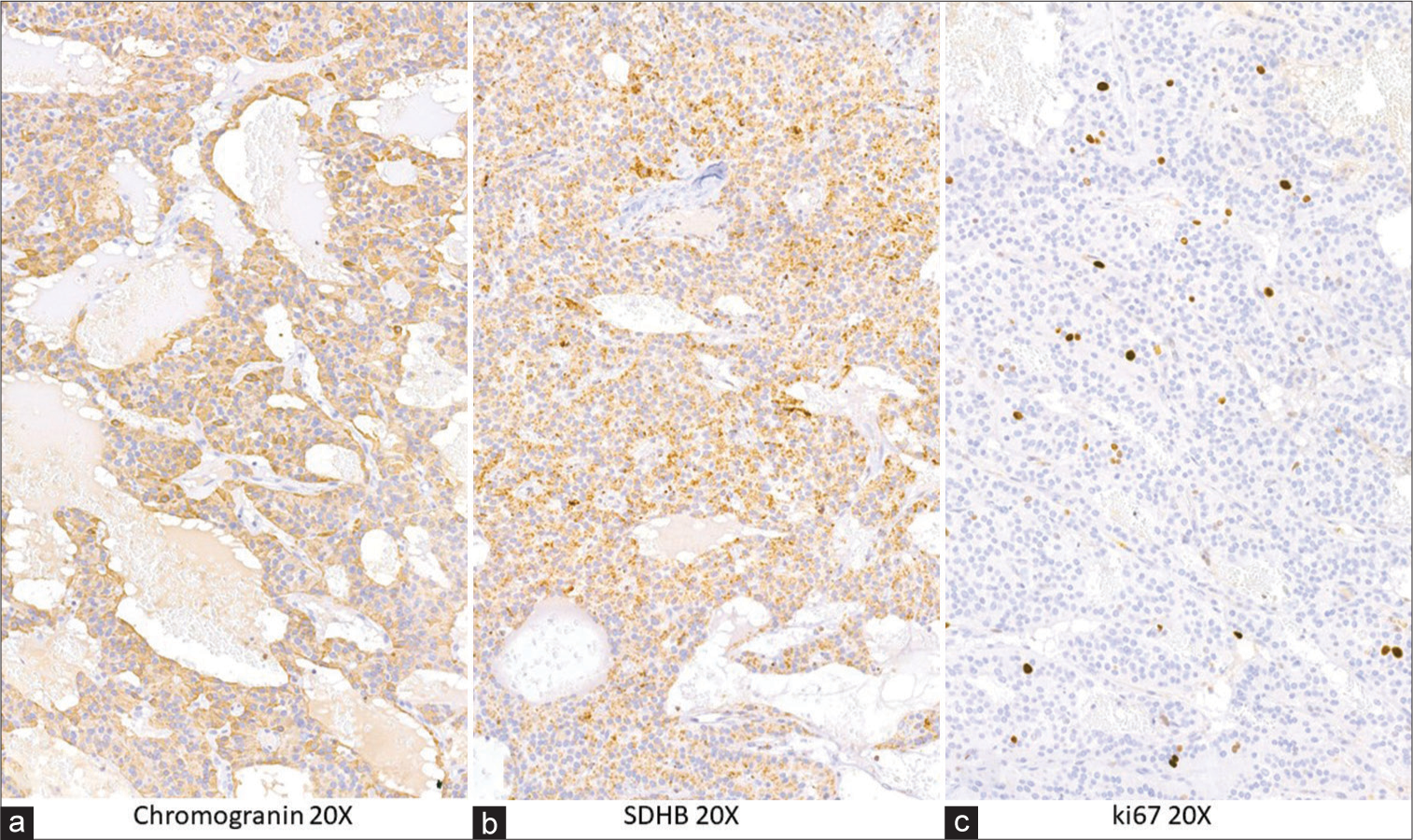
Neoplastic cells were immunoreactive for synaptophysin and chromogranin-A, confirming their neuroendocrine differentiation [
Patient 2
Intraoperatively, the lesion appeared gray and was of a hard consistency.
Grossly, the specimen was a 1.7 cm encapsulated oval and brown nodular mass.
Histopathological examination showed a well-circumscribed nodule composed of nests of monomorphic round cells and a prominent thin vascular network with congested vessels. Capsular invasion and necrosis were not observed [
Immunohistochemical studies were performed, and tumor cells were positive for chromogranin [
DISCUSSION
Spinal PGs are rare, with an incidence in the general population of circa 0.07/100,000 inhabitants.
Shtaya et al.[
Diagnosis for intradural lesions is based on MRI. As imaging findings are non-specific,[
On an endocrinological basis, PGs are classified as secretory and non-secretory neoplasia’s. There have been a few documented cases in the literature of PG symptomatology marked by sympathetic hyperactivity, such as paroxysmal hypertension and metabolic disorders.[
The major concern of PGs is that they can cause lethal hypertensive crises through excessive catecholamine-release. Symptoms can vary and be unspecific, such as visual disturbances, increased heart rate, headache, and vomiting. Therefore, a high degree of suspicion is critical for prompt diagnosis and management. Of primary concern is BP control, for example, through bolus administration or continuous infusion of vasodilators such as urapidil, labetalol, or nitroglycerine. Other options are the application of phentolamine, a long-acting, adrenergic, and alpha-receptor blocking agent given as an intravenous bolus of 2.5–5 mg at the rate of 1 mg/min and which can be repeated every 3–5 min. To reduce the risk of an intraoperative hypertensive crisis, proper preoperative management focusing on BP adjustment and sufficient blood volume to assure a hemodynamic stable patient is paramount. Preparation of patients, especially for catecholamine-producing tumors and cardiovascular assessment, is a cornerstone of this surgery. The objective of the preparation is to limit preoperative hypertension to 160/90 mmHg and the vasoconstrictive, tachycardic effects of catecholamine. The tumor’s size (>4 cm), the level of catecholamines secreted, a mean preoperative pressure >100 mmHg, and hypovolemia are factors leading to cardiovascular instability.
If manipulated during surgical excision, they could theoretically release neurotransmitters and provoke sudden abnormal BP variations and complications such as cerebrovascular accidents or pulmonary edema.[
From a histopathological point of view, these tumors may also show an uncommon immunohistochemical profile concerning cytokeratin expression; in our case, tumor cells were positive for this epithelial marker.
It is well known that most PGs are not immunoreactive for cytokeratin, a feature allowing differentiation from other neuroendocrine neoplasms; positive cytokeratin staining is a rare occurrence, as supported by Dermawan et al.[
Given the possibility of neurosecretion, subtle endocrine symptoms have to be considered when PGs are suspected. During capsule coagulation, we noted a slight peak of BP, very likely the consequence of catecholamine release by the tumor. We achieved a total removal without the need for debulking.
CONCLUSION
When suspecting PGs, the biochemical work-up should include the determination of free metanephrine or urinary metanephrine, urinary adrenaline, and noradrenaline. Furthermore, 24 h BP monitoring should be considered. If laboratory tests are suggestive of a secretory tumor, surgery should include an anesthesiologic preparation similar to cases of pheochromocytoma.[
Take-home points
PGs are very rare types of neuroendocrine tumors PGs may be endocrinologically active, containing neurotransmitters Preoperative preparation should focus on adequate BP adjustment and blood volume status aiming at a hemodynamically stable patient The risk of not recognizing active PGs can have serious intraoperative consequences.
Data availability statement
All data related to these two cases are mentioned in the manuscript and can also be requested directly by the corresponding author, Dr. Zekaj.
Ethical approval
Not applicable.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
We thank Dr. Fabrice Michaut, an anesthetist, Paris, France for reviewing the manuscript and his constructive comments.
References
1. Chetty R, Pillay P, Jaichand V. Cytokeratin expression in adrenal phaeochromocytomas and extra-adrenal paragangliomas. J Clin Pathol. 1998. 51: 477-8
2. Dermawan JK, Mukhopadhyay S, Shah AA. Frequency and extent of cytokeratin expression in paraganglioma: An immunohistochemical study of 60 cases from 5 anatomic sites and review of the literature. Hum Pathol. 2019. 93: 16-22
3. Fang F, Ding L, He Q, Liu M. Preoperative management of pheochromocytoma and paraganglioma. Front Endocrinol (Lausanne). 2020. 11: 586795
4. Gunawardane PT, Grossman A. Phaeochromocytoma and paraganglioma. Adv Exp Med Biol. 2017. 956: 239-59
5. Jeffs GJ, Lee GY, Wong GT. Functioning paraganglioma of the thoracic spine: Case report. Neurosurgery. 2003. 53: 992-4
6. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021. 23: 1231-51
7. Méndez JC, Carrasco R, Prieto MA, Fandiño E, Blázquez J. Paraganglioma of the cauda equina: MR and angiographic findings. Radiol Case Rep. 2019. 14: 1185-7
8. Miliaras GC, Kyritsis AP, Polyzoidis KS. Cauda equina paraganglioma: A review. J Neurooncol. 2003. 65: 177-90
9. Nowacki N, Roth R, Iwenofu OH. Diffuse cytokeratin positivity in an intradural paraganglioma of the lumbar vertebra: A diagnostic pitfall!. Appl Immunohistochem Mol Morphol. 2016. 24: e22-4
10. Pipola V, Boriani S, Bandiera S, Righi A, Barbanti Bròdano G, Terzi S. Paraganglioma of the spine: A twenty-years clinical experience of a high volume tumor center. J Clin Neurosci. 2019. 66: 7-11
11. Shtaya A, Iorga R, Hettige S, Bridges LR, Stapleton S, Johnston FG. Paraganglioma of the cauda equina: A tertiary centre experience and scoping review of the current literature. Neurosurg Rev. 2022. 45: 103-18
12. Turk O, Yaldiz C, Antar V, Batur S, Demirel N, Atci B. Spinal paragangliomas: Surgical treatment and follow-up outcomes in eight cases. Medicine (Baltimore). 2018. 97: e12468