- Department of Neurosurgery, University of Texas MD Anderson Cancer Center, Houston, United States
- Department of Radiation Physics, University of Texas MD Anderson Cancer Center, Houston, United States
- Department of Radiology, University of Texas MD Anderson Cancer Center, Houston, United States
Correspondence Address:
Robert Y. North, Department of Neurosurgery, University of Texas MD Anderson Cancer Center, Houston, United States.
DOI:10.25259/SNI_384_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Romulo Augusto Andrade de Almeida1, Francisco Call-Orellana1, Christopher C. Young1, Franco Rubino1, Sara L. Thrower2, Stephen R. Chen3, Robert Y. North1. Hybrid open-endovascular onyx embolization of spinal type IVb perimedullary spinal arteriovenous fistula through direct posterior spinal vein access: A case report. 27-Sep-2024;15:343
How to cite this URL: Romulo Augusto Andrade de Almeida1, Francisco Call-Orellana1, Christopher C. Young1, Franco Rubino1, Sara L. Thrower2, Stephen R. Chen3, Robert Y. North1. Hybrid open-endovascular onyx embolization of spinal type IVb perimedullary spinal arteriovenous fistula through direct posterior spinal vein access: A case report. 27-Sep-2024;15:343. Available from: https://surgicalneurologyint.com/surgicalint-articles/13123/
Abstract
Background: Spinal arteriovenous fistulas (SAVFs) are direct communication between arteries and veins without intervening abnormal vessel nidus, which often results in venous congestion and spinal cord dysfunction. Ventrally located SAVF can be challenging to treat through traditional open or endovascular approaches.
Case Description: We describe a hybrid (open/endovascular) procedure in a 72-year-old male with a Takai Type IVb SAVF presenting with paraparesis and sphincter dysfunction. Imaging revealed a conus medullaris SAVF in which the main fistulous connection was located ventrally. The conventional endovascular approach was deemed risky, and open surgery failed in the first attempt. The SAVF was resolved using a hybrid approach: under direct visualization, an engorged dorsal vein was punctured with an Angiocath, and a fluoroscopy-guided microcatheter was advanced through it to reach and embolize the ventral perimedullary fistulous connection. After surgery, his progressive neurological decline stabilized, radiographic spinal cord edema improved, and follow-up angiography confirmed obliteration of the fistula. Neurological function remained at the preoperative baseline.
Conclusion: This approach may be a treatment for selected cases of type IVb SAVF. Easily accessible feeding vessels are coagulated and cut; the inaccessible ones can be embolized endovascularly during the same procedure.
Keywords: Case report, Embolization, Endovascular, Hybrid, Spinal arteriovenous fistula, Spine
INTRODUCTION
Spinal arteriovenous fistulas (SAVFs) are direct communication between arteries and veins without intervening abnormal vessel nidus, which often results in venous congestion and spinal cord dysfunction.[
CLINICAL PRESENTATION
A 72-year-old man presented with a history of hypertension, cirrhosis (Child-Pugh A), pituitary macroadenoma, status post-endoscopic endonasal resection complicated by postoperative hemorrhage, and multiple failed attempts at lumbar drain placement who had progressive gait ataxia, back pain, urinary retention, and headaches. Spine magnetic resonance imaging revealed a low-lying conus ending at L3–4, multiple intradural cysts suggestive of arachnoid adhesions, and abnormal vessels suggestive of a SAVF [
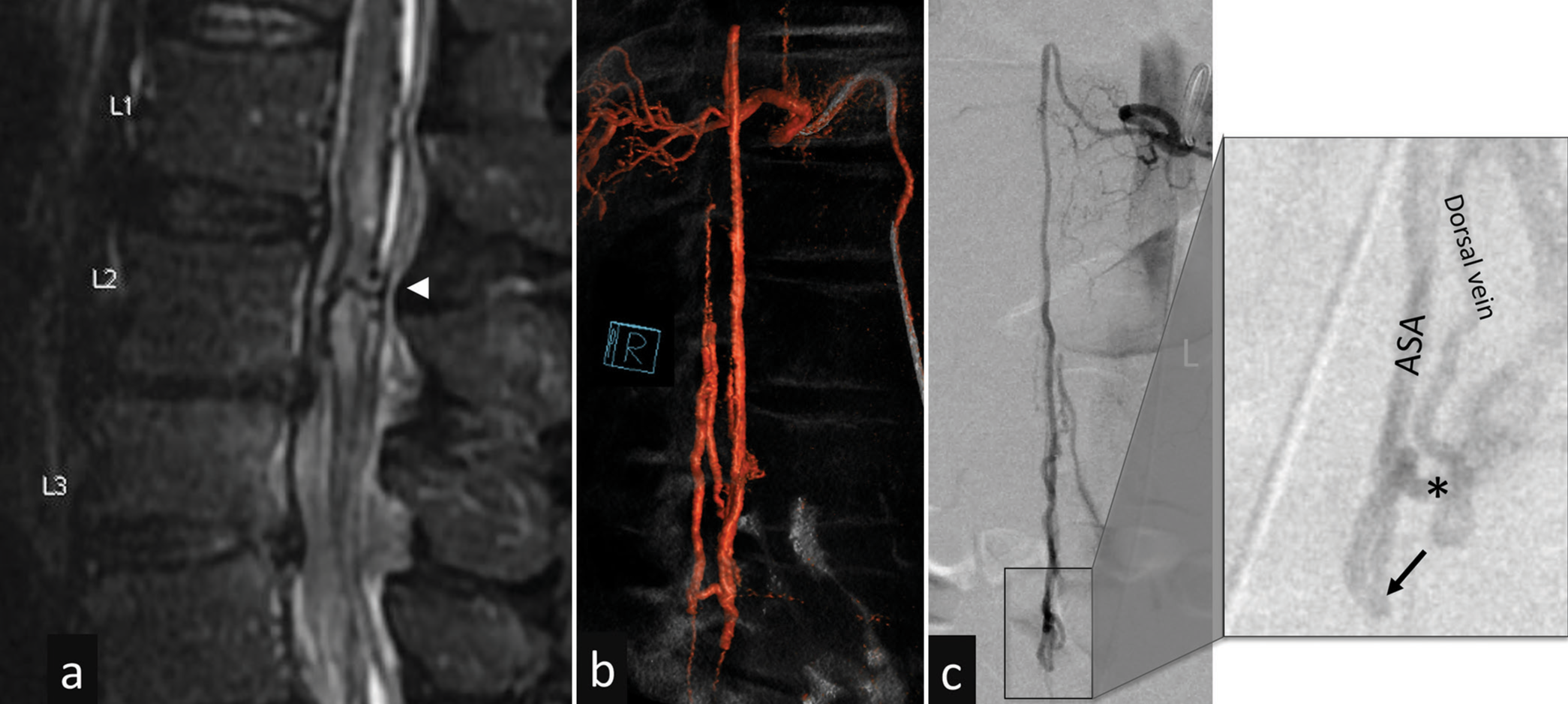
Figure 1:
Preoperative images (a) T2-weighted sagittal MRI scan shows an edematous conus medullaris with a large vein crossing through the parenchyma at the level of L2 (arrowhead). (b and c) Spinal angiogram 3D reconstruction and original preoperative images. The zoom-in inset image, at a slight oblique angle, provides information on the anatomy – the dorsal vein is quickly filled with blood from main fistulous connection (black arrow) with anterior spinal artery (ASA). Smaller, superficial, feeders are not seen. The asterisk points at where the clip was applied – the intense adhesions and vessel tortuosity prevented proper clip position, which remained at a superficial (dorsal) location.
Given progressive neurological symptoms, treatment was recommended. Endovascular treatment was not attempted due to the unclear location of the fistulous point and the high risk of spinal cord ischemia from direct anterior spinal artery catheterization. Surgical treatment was undertaken with laminectomies from T12 to L2 as possible fistula connections were noted at T12–L1 junction and L2. Extensive arachnoid adhesions involving the spinal cord circumferentially, nerve roots, and spinal cord vasculature were encountered, possibly related to prior hemorrhage and lumbar drain placement. After the dissection of adhesions there was a large, dorsal, arterialized vein clearly visualized. Attention was focused on the two areas seen on angiography as possible sites of fistulous connections: T12–L1 lateral cord and L2 ventral cord. At T12–L1, a tortuous nest of small vessels, apparently coming from the posterior spinal artery along the dorsal roots and lateral pia that connected to the dorsal vein, was noted. A temporary clip was applied across these vessels without change in neuromonitoring and these were cauterized and cut. The numerous and dense adhesions impaired attempts to rotate the spinal cord and directly visualize the ventral cord. A dilated vein traversing the spinal cord [
Immediately following surgery patient had transient proximal left leg weakness that improved quickly to a 4/5 strength. However, on the 7th postoperative day, there was a new worsening, with bilateral, distal-predominant low extremity weakness. MRI demonstrated worsening spinal cord edema [
Figure 2:
Post clipping images (a) T2-weighted sagittal MRI scan after initial fistula treatment demonstrates increased cord edema extension (arrows). (b) Control angiogram demonstrated recruitment of multiple anterior medullary veins after clipping (black arrow). (c) These recruited veins, easier viewed at a higher magnification, are pointed out by the white arrows.
The patient underwent a hybrid open-endovascular approach involving the removal of a previously placed clip on the hypertrophied vein and puncture of the large dorsal vein with a 20-gauge Angiocath. A 1.3 French Headway Duo 167 microcatheter (Microvention Inc, Aliso Viejo, CA, USA) was advanced over a Chikai 008 microwire (Asahi Intecc USA, Tustin, CA, USA) and retrograde access was obtained through wire recanalization of the thrombosed, previously clipped vein [
Figure 3:
Intra and postembolization angiograms and MRI. (a) Intraprocedural angiogram shows the advancement of the catheter through the thrombosed segment after clip removal. (b) Immediate post onyx embolization demonstrating occlusion of the feeding vessels with white arrow pointing to the puncture site in the dorsal vein. (c) Intraoperative angiogram immediate post-embolization. (d) MRI after revision hybrid open-endovascular treatment demonstrates decreased spinal cord edema (arrows). (e and f) 1-month follow-up angiography with stable findings and no arteriovenous shunting.
DISCUSSION
In this case, open surgical access provided a route for surgical closure of dorsal and laterally located fistulous connections and provided successful transvenous endovascular access to the ventral feeder [
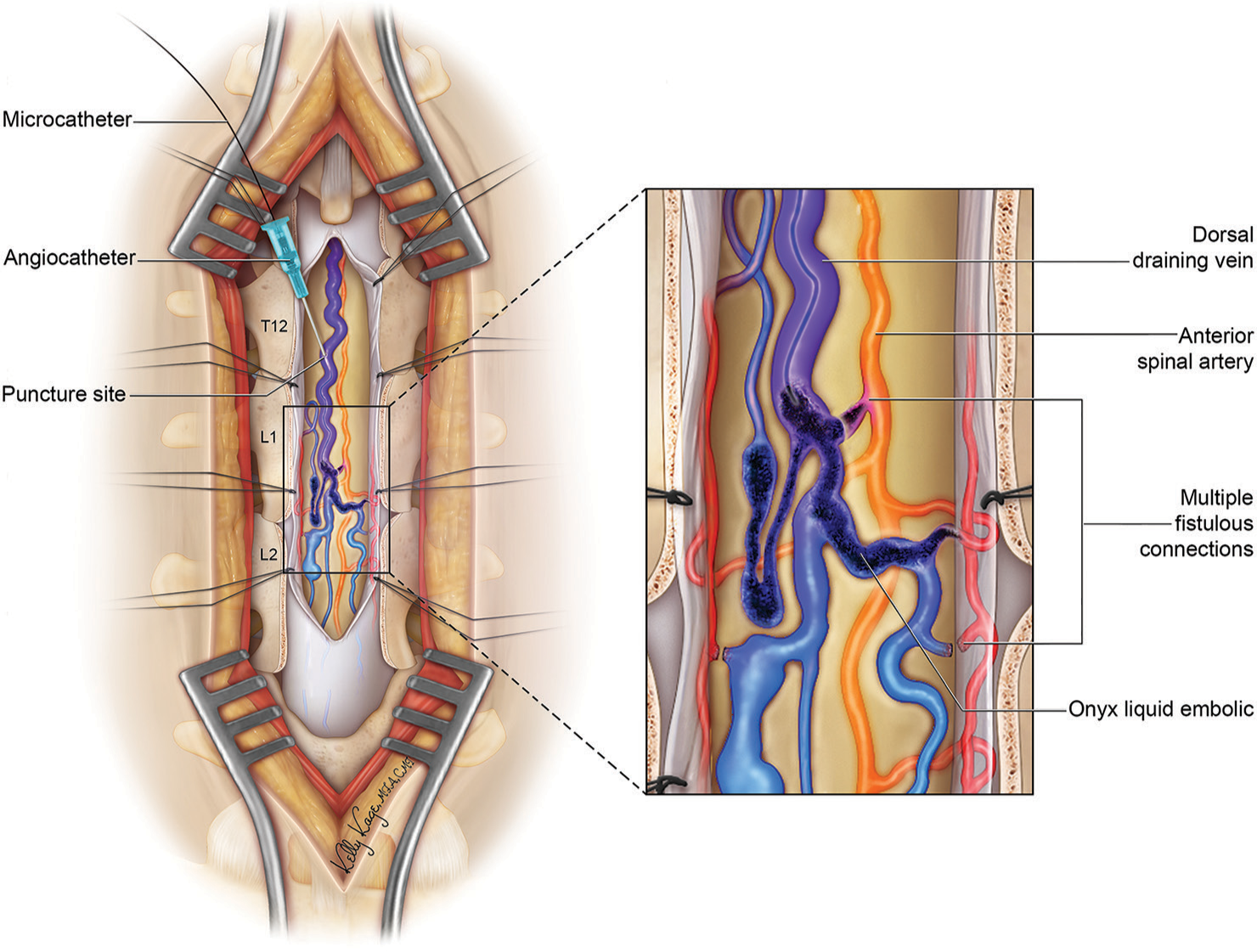
Figure 4:
Artistic representation of the hybrid approach to the spinal arteriovenous fistula. In this representative case, based on the actual case here presented, the spinal cord is exposed through a standard posterior approach. Dorsal and superficial feeders can be coagulated and cut. Deep feeders, crossing through the spinal cord, can be reached by the advance of microcatheter through the engorged recipient dorsal vein and occluded with liquid embolic.
CONCLUSION
This hybrid approach may be a treatment for selected cases of type IVb SAVF. The multiple feeding vessels can be addressed according to their accessibility: easily surgically accessible ones are coagulated and cut, whereas the inaccessible ones can be endovascularly accessed and embolized during the same procedure.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We thank Kelly Kage, who was the illustrator of Figure 4.
References
1. Anson JA, Spetzler RF. Classification of spinal AVM and implication for treatment. BNI Q. 1992. 8: 2-8
2. Casasco A, Guimaraens L, Cuellar H, Theron J, Heredero J. Direct percutaneous venous puncture and embolization of giant perimedullary arteriovenous fistulas. AJNR Am J Neuroradiol. 2011. 32: E10-3
3. Cho KT, Lee DY, Chung CK, Han MH, Kim HJ. Treatment of spinal cord perimedullary arteriovenous fistula: Embolization versus surgery. Neurosurgery. 2005. 56: 232-41
4. Di Chiro G, Doppman JL, Ommaya AK. Radiology of spinal cord arteriovenous malformations. Prog Neurol Surg. 1971. 4: 329-54
5. Flores BC, Klinger DR, White JA, Batjer HH. Spinal vascular malformations: Treatment strategies and outcome. Neurosurg Rev. 2017. 40: 15-28
6. Geibprasert S, Pereira V, Krings T, Jiarakongmun P, Toulgoat F, Pongpech S. Dural arteriovenous shunts: A new classification of craniospinal epidural venous anatomical bases and clinical correlations. Stroke. 2008. 39: 2783-94
7. Heros RC, Debrun GM, Ojemann RG, Lasjaunias PL, Naessens PJ. Direct spinal arteriovenous fistula: A new type of spinal AVM. Case report. J Neurosurg. 1986. 64: 134-9
8. Kienzler JC, Schoepf S, Marbacher S, Diepers M, Remonda L, Fandino J. Intraoperative spinal angiography during microsurgical occlusion of spinal dural arteriovenous fistula within the hybrid operation room. J Neurol Surg A Cent Eur Neurosurg. 2022. 83: 486-93
9. Mourier KL, Gobin YP, George B, Lot G, Merland JJ. Intradural perimedullary arteriovenous fistulae: Results of surgical and endovascular treatment in a series of 35 cases. Neurosurgery. 1993. 32: 885-91
10. Patsalides A, Knopman J, Santillan A, Tsiouris AJ, Riina H, Gobin YP. Endovascular treatment of spinal arteriovenous lesions: Beyond the Dural fistula. AJNR Am J Neuroradiol. 2011. 32: 798-808
11. Rangel-Castilla L, Holman PJ, Krishna C, Trask TW, Klucznik RP, Diaz OM. Spinal extradural arteriovenous fistulas: A clinical and radiological description of different types and their novel treatment with Onyx. J Neurosurg Spine. 2011. 15: 541-9
12. Riché MC, Melki JP, Merland JJ. Embolization of spinal cord vascular malformations via the anterior spinal artery. AJNR Am J Neuroradiol. 1983. 4: 378-81
13. Rodesch G, Hurth M, Alvarez H, Tadié M, Lasjaunias P. Classification of spinal cord arteriovenous shunts: Proposal for a reappraisal--the Bicêtre experience with 155 consecutive patients treated between 1981 and 1999. Neurosurgery. 2002. 51: 374-9 discussion 379-80
14. Rosenblum B, Oldfield EH, Doppman JL, Di Chiro G. Spinal arteriovenous malformations: A comparison of Dural arteriovenous fistulas and intradural AVM’s in 81 patients. J Neurosurg. 1987. 67: 795-802
15. Shin HK, Suh DC, Jeon SR. Intraoperative direct puncture and embolization (IOPE) using a glue material for spinal cord arteriovenous fistula: A case report. Eur Spine J. 2015. 24: S594-9
16. Spetzler RF, Detwiler PW, Riina HA, Porter RW. Modified classification of spinal cord vascular lesions. J Neurosurg. 2002. 96: 145-56
17. Takai K. Spinal arteriovenous shunts: Angioarchitecture and historical changes in classification. Neurol Med Chir (Tokyo). 2017. 57: 356-65
18. Zhang N, Xin WQ. Application of hybrid operating rooms for treating spinal dural arteriovenous fistula. World J Clin Cases. 2020. 8: 1056-64