- Department of Neurosurgery, Hospital de Especialidades N° 71, Instituto Mexicano del Seguro Social, Torreón Coahuila,
- Department of Neurosurgery, Hospital de Especialidades, Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social, México City, México,
- Department of Pediatrics, School of Medicine, Stanford University, Stanford, California, United States,
- Department of Pediatric Neurosurgery, Children’s Hospital of Mexico “Federico Gómez”, Mexico City,
- Department of Health Education and Research, Hospital de Especialidades N° 71, Instituto Mexicano del Seguro Social, Torreón Coahuila,
- Department of Anatomycal Pathology, Hospital de Especialidades N° 71, Instituto Mexicano del Seguro Social, Torreón Coahuila,
- Department of Health Education, Hospital de Especialidades N° 71, Instituto Mexicano del Seguro Social, Torreón Coahuila,
- Department of Pediatric Infectious Disease, Hospital de Pediatría, Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social, México City, Mexico.
Correspondence Address:
María F. De la Cerda-Vargas
Department of Pediatric Infectious Disease, Hospital de Pediatría, Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social, México City, Mexico.
DOI:10.25259/SNI_895_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: María F. De la Cerda-Vargas1, B. A. Sandoval-Bonilla2, James M. McCarty3, Fernando Chico-Ponce De León4, José A. Candelas-Rangel1, Jorge D. Rodríguez-Rodríguez1, Pedro Navarro-Domínguez1, Melisa A. Muñoz- Hernández5, Elizabeth Meza-Mata6, Elena M. Fernández-González7, Mariana G. Sámano-Aviña8. Hydrocephalus in Mexican children with Coccidioidal Meningitis: Clinical, serological, and neuroimaging findings. 24-Mar-2021;12:119
How to cite this URL: María F. De la Cerda-Vargas1, B. A. Sandoval-Bonilla2, James M. McCarty3, Fernando Chico-Ponce De León4, José A. Candelas-Rangel1, Jorge D. Rodríguez-Rodríguez1, Pedro Navarro-Domínguez1, Melisa A. Muñoz- Hernández5, Elizabeth Meza-Mata6, Elena M. Fernández-González7, Mariana G. Sámano-Aviña8. Hydrocephalus in Mexican children with Coccidioidal Meningitis: Clinical, serological, and neuroimaging findings. 24-Mar-2021;12:119. Available from: https://surgicalneurologyint.com/surgicalint-articles/10661/
Abstract
Background: Coccidioidal meningitis (CM) is a fungal infectious disease that rarely affects children. Even in endemic areas, coccidiomycosis rarely affects the pediatric population. However, 40% of affected children develop hydrocephalus. Here, we describe the clinical, serological, and neuroimaging findings in a series of Mexican children admitted to our neurosurgical service with hydrocephalus and subsequently diagnosed with CM.
Methods: We report a prospective series of pediatric patients with hydrocephalus secondary to CM in an endemic area at the north of Mexico. Our report includes children with CM who were hospitalized from 2015 to 2019 in a regional hospital in Torreón, Coahuila. Clinical evolution was monitored for 1 year after hospital discharge.
Results: Our series include five children with CM (2–17-years-old, three female), who were hospitalized for hydrocephalus and developed intracranial hypertension. The most frequent neuroimaging findings were leptomeningeal enhancement (5/5) and basal arachnoiditis (4/5), followed by asymmetric hydrocephalus (3/5), abnormalities in fourth ventricle morphology (3/5), and cerebral vasculitis (2/5). CM was diagnosed by positive serology or pathology studies. All children were initially managed with fluconazole and a shunt was placed for management of hydrocephalus. Four patients recovered without permanent neurological deficits and one subject developed persistent vegetative state. One year after hospital discharge, none of the subjects died.
Conclusion: This series contributes to the limited number of pediatric CM cases reported in the literature, and describes neuroimaging findings in the pediatric population. The cases here presented show that the identification of Coccidioides as causal agent in pediatric meningitis is crucial for targeted treatment and can affect dramatically neurological prognosis. Furthermore, our report stresses that even in endemic areas pediatric coccidiomycosis represents a diagnostic challenge, which is further exacerbated by the limited availability of resources in these regions. Therefore, a positive immunoglobulin G by enzyme immunoassay is enough for diagnosis of CM in endemic areas without access to CF.
Keywords: Coccidioidal meningitis, Diagnosis, Hydrocephalus, Pediatric, Serology
INTRODUCTION
Coccidioidomycosis is a fungal disease with pulmonary origin[
Given that more than 90% of untreated patients die within the 1st year,[
TCD4+ cells perform an important role in the defense against invasive fungal infection.[
Specific genetic mutations that alter the immune response involving Interferon-γ/Interleukin-12 and other cellular immune pathways may predispose to severe Coccidioidomycosis. Patients with these mutations have a higher incidence of disseminated infection by Coccidioides sp. in comparison to the healthy population in an endemic area (75% vs. 1%).[
The objective of this study was to describe the clinical, radiological, and serological findings in a series of pediatric patients with hydrocephalus secondary to CM in our hospital.
MATERIAL AND METHODS
Pediatric patients with hydrocephalus secondary to CM diagnosed at our center (Hospital de Especialidades N° 71 Mexican Social Security Institute) between January 2015 and January 2019 were included in the study. Our hospital is a regional center in northern Mexico, an endemic region for coccidioidomycosis.
We conducted a prospective case series. Hydrocephalus was diagnosed by clinical signs and simple cranial computed tomography (CT) scan. All the subjects underwent gadolinium-enhanced magnetic resonance imaging (MRI) 1 month after surgery. CM was diagnosed by a positive serology test (IgG in CSF ≥ 0.150 D.0.) performed by enzyme immunoassay (EIA), or by isolation of C. immitis in a leptomeningeal biopsy, CSF culture or CSF cytology. Furthermore, IgG and IgM levels against C. immitis were measured in serum. Patients with CM underwent serological tests for HIV, Hepatitis B and C. Ig levels (IgA, IgE, IgG, and IgM) were also measured to rule out secondary and humoral immunodeficiencies; blood lymphocytes levels were tested in only one case. As part of the differential diagnosis, a polymerase chain reaction (PCR) for tuberculosis in CSF was performed in all subjects.
Approval for this study was obtained from the Institutional Review Board of our Center (R-2020-501-020). Before enrollment, informed consent was signed by parents or tutors of the patients.
RESULTS
Five pediatric patients (including three female and two male) with ages ranged from 2 to 17 years old (average 9-years old). At admission all the patients presented hydrocephalus, and a ventriculoperitoneal shunt (VPS) was subsequently placed. Physical examination revealed low weight in one subject and moderate malnutrition in two cases [
Clinical manifestations
All patients showed signs of endocranial hypertension on admission. Sixth nerve palsy was identified in three children and central facial palsy in one case. Seizures were documented in one child, while in another case the patient developed ataxic gait. Three children debuted with hemiparesis; two patients had Parinaud’s sign and meningeal signs on admission. Papilledema was found in three patients on fundoscopy. Fever was recorded in only three cases [
Neuroimaging findings [ Table 3 ]
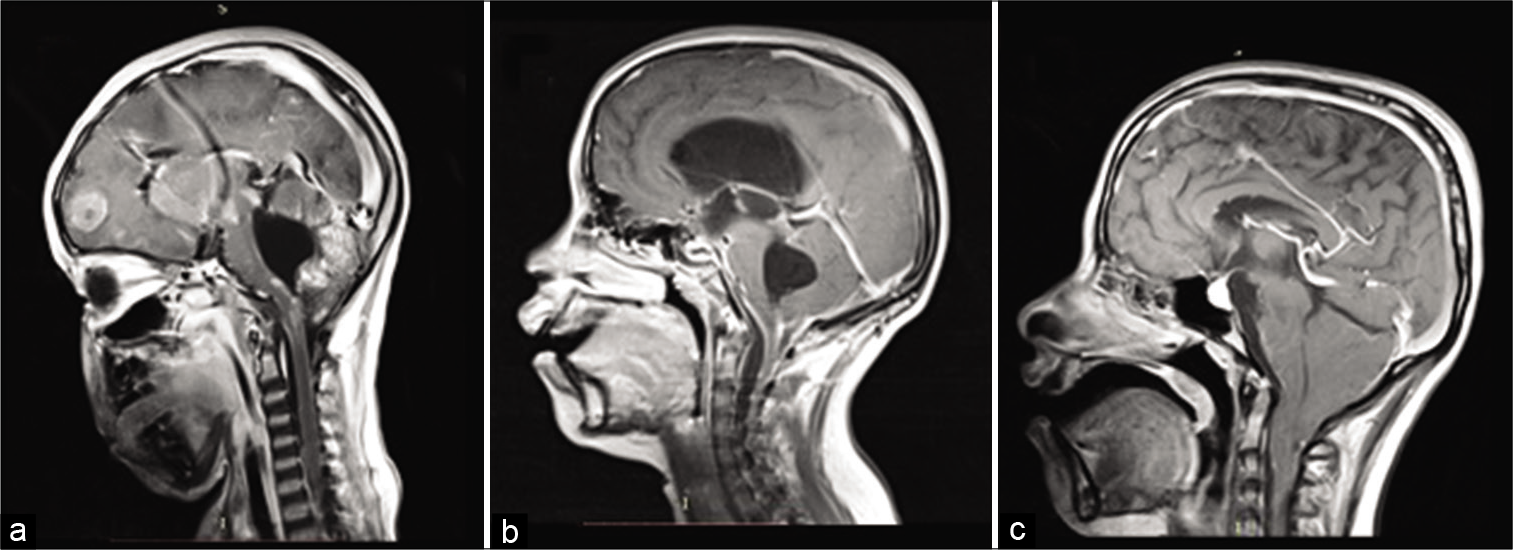
Preoperative CT scan showed an Evans´ index greater than postoperative control MRI (average of 0.39, ranges 0.31–0.58, and 0.30, ranges 0.15–0.31, respectively). Three cases presented asymmetric hydrocephalus (AH) [
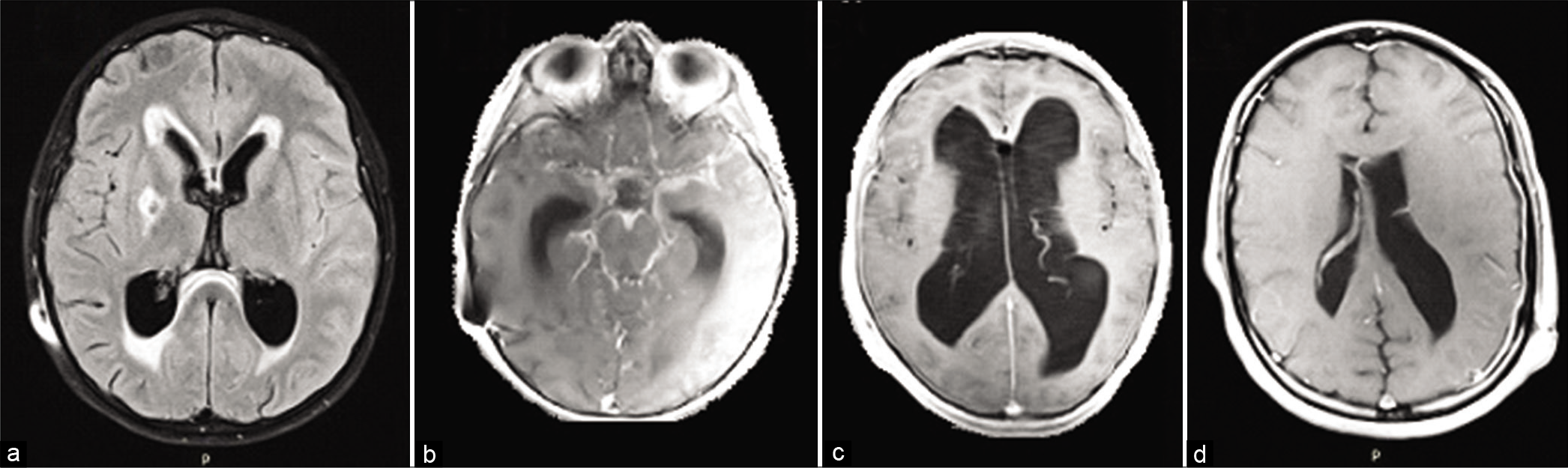
Figure 1:
(a) T2-FLAIR-weighted magnetic resonance imaging (MRI) showing ischemic stroke in the right head of the caudate nucleus and anterior limb of the internal capsule, (b) Gadolinium-enhanced T1-weighted MRI with basal arachnoiditis, (c) Gadolinium-enhanced T1-weighted MRI showing asymmetric hydrocephalus (AH) and leptomeningeal enhancement, (d) Gadolinium T1-weighted MRI showing AH and left frontoparietal leptomeningeal enhancement.
Serological and histopathological studies [ Table 4 ]
Serological tests in CSF revealed IgG levels from 0.150 to 0.495 D.0. (mean 0.845 D.0. across subjects) while in serum IgG titers ranged from 0.005 to 2.16 D.0. (mean 0.8344). Children with CM diagnosed by serology presented serum IgG levels against Coccidioides >0.500 D.0 with an upper limit of 2.16 D.0. (case 1, 3-4). In contrast, in patients diagnosed by histopathology (cases two and five) titers were <0.150 D.0. and therefore considered negative for systemic coccidiomycosis. Only two patients, with serological diagnosis of CM, presented positive serum IgM titers for Coccidioides (≥0.150 D.0)*.
Mean IgE levels ranged from 25 to 173.UI/ml (mean 0.845 D.0.). Case 5 (patient with Chiari II malformation, who underwent myelomeningocele repair at birth), presented high levels of IgE but did not meet the criteria for Job Syndrome. One patient presented high levels of IgM and in 40% (n = 2) high levels of IgG were observed. The average IgG titers ranged from 1219 to 1858 mg/dl (mean 1522.4 mg/dl). Despite having the highest serum IgG titers, Case 4 antibodies against Coccidioides were not increased (negative test). Hyper or hypogammaglobulinemia was not detected in the rest of cases. T lymphocyte counts were normal in the tested case. No patient presented with eosinophilia or lymphopenia. Serology tests for HIV, hepatitis B and C were negative in all cases.
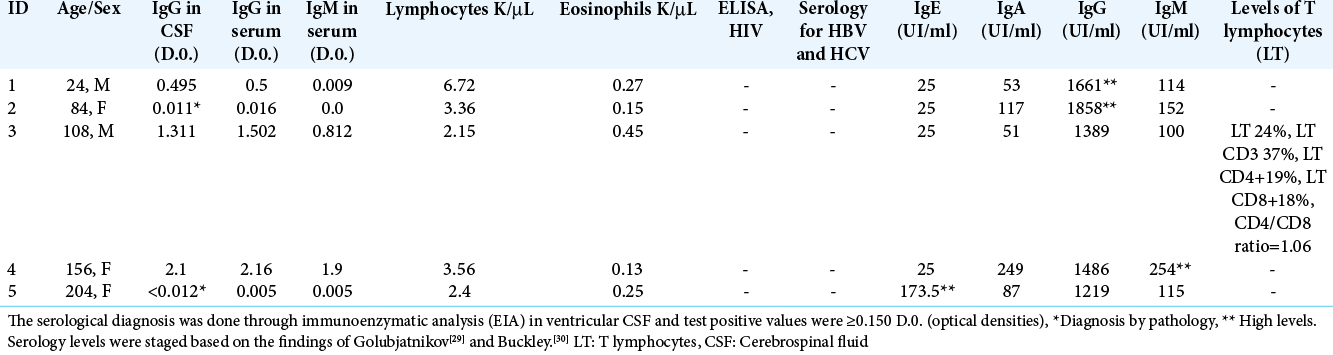
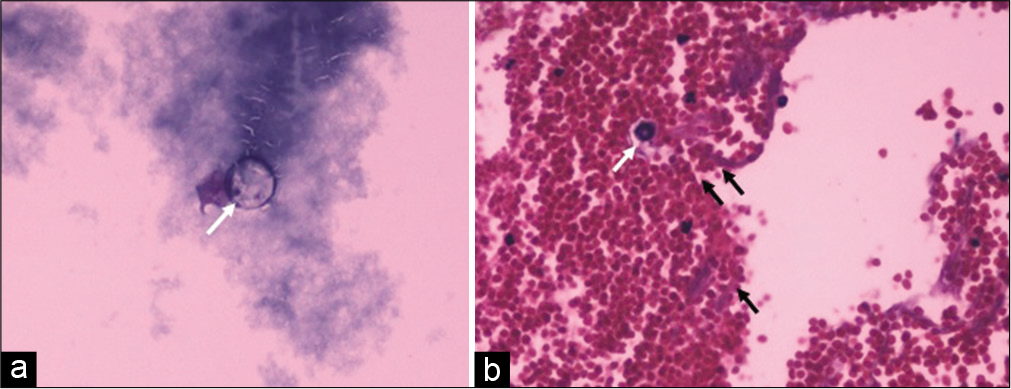
CSF cytology was positive in two children [
Medical treatment
Fluconazole at doses of 6–12 mg/kg/day was prescribed as life-long antifungal therapy. Corticosteroids were indicated in case of cerebral vasculitis, or signs of serious illness. Case 2 was treated with caspofungin and cephalosporins initially due to a positive culture for Pseudomonas aeruginosa in the distal shunt. The child presented neurological deterioration and poor evolution and MRI showed HA, cerebral vasculitis, and IFV. After a month of hospitalization and multiple shunt replacements, C. immitis was isolated in ventricular CSF [
Surgical procedures
VPS placement was performed in all subjects. In case of bacterial superinfection, external ventricular drains were also placed (case 2). An average of three surgeries was performed per subject (range 1–9). Case 2 developed IFV and it was therefore decided to place a shunt of the fourth ventricle into the subarachnoid space.
Follow-up
Case 2 presented hyponatremia secondary to transient syndrome of inappropriate antidiuretic hormone secretion (SIHAD), with spontaneous resolution. Cases one and three were initially treated as tuberculous meningitis, but CSF PCR was negative for tuberculosis, and CM was later diagnosed by positive IgG serology against Coccidioides in ventricular CSF. These two children developed rash and drug related hepatitis due to the interaction of fluconazole and the initial antifimic therapy. The average follow-up was 8 months after discharge. Serum IgG and IgM titers against Coccidioides and liver function tests were periodically requested. Control serological tests were reported as negative (<0.150 D.0.). Clinical outcomes were satisfactory in four cases, without evidence of long-term neurological compromise. However, one child (case two) developed a persistent vegetative state. One year after discharge none of the subjects died.
DISCUSSION
According to current Centers for Disease Control and Prevention guidelines, the endemic regions of coccidioidomycosis are found in the Southwestern USA, South-Central Washington state, Northern Mexico, and areas in central and South America. However, Mexico represents the country with the most reported cases in Latin America, with a prevalence of 10–40%.[
We aimed to describe the clinical, radiological, and serological findings in pediatric patients with hydrocephalus due to meningeal coccidioidomycosis in our hospital.
Clinical findings
Headache is the most common symptom documented in CM. Altered personality, impaired cognition, and alterations in the state of consciousness, as well as neurological focal signs (mainly vision disturbances) are symptoms related with severity in CM.[
Neuroimaging findings
In studies reported from 1981[
Shetter et al.[
Shetter et al.[
Serological studies
The diagnosis of CM is currently made by detection of serum IgG antibodies against Coccidioides by complement fixation (CF) test along with clinical manifestations and CSF findings suggestive of CM (eosinophilic pleocytosis, protein level >150 mg/dL and low glucose level);[
Innate and adaptive immunity plays a critical role in host defense against systemic mycoses. Specifically, TCD4+ lymphocytes that differentiate into Th1 and Th17 are essential in the response against severe Coccidioidal disease.[
Few studies have investigated immune factors and predisposing conditions in pediatric subjects with coccidiomycosis. Mendel et al.[
Few pediatric cases with CM have been published. About 1% of pediatrics with coccidioidomycosis are expected to have disseminated disease. The difference between the disease in adults and pediatric patients is unknown, however, is expected a more serious disease in children and often associated with some hereditary or acquired immunodeficiency, while in adults AIDS, pregnancy, and immunocompromised (transplant donors) are well-established risk factors. Despite the efforts for early diagnosis and treatment in children, the IDSA guidelines for coccidioidomycosis (2016) are focused on the adult and neonatal population, and pediatrics have been managed on the criteria established for adults.[
CONCLUSION
This report is one of the few on pediatric subjects with CM in Mexico. In our series, the most common neuroimage findings were hydrocephalus (5/5) and leptomeningeal enhancement (5/5), followed by basal arachnoiditis (3/5), AH (3/5), abnormalities of the fourth ventricle (3/5), and cerebral vasculitis (2/5). The findings here described support the claim that, in endemic areas without access to CF, a positive IgG in CSF or serum detected by EIA can be useful to diagnose CM.