- Department of Neurosurgery, Tokai University Hachioji Hospital, Ishikawa Machi, Hachioji, Tokyo, Japan.
Correspondence Address:
Masami Shimoda, Department of Neurosurgery, Tokai University Hachioji Hospital, Ishikawa Machi, Hachioji, Tokyo, Japan.
DOI:10.25259/SNI_1023_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masaaki Imai, Masami Shimoda, Shinri Oda, Kaori Hoshikawa, Takahiro Osada, Rie Aoki, Azusa Sunaga. Hyperintense posterior cerebral artery sign in patients with reversible cerebral vasoconstriction syndrome. 16-Nov-2021;12:558
How to cite this URL: Masaaki Imai, Masami Shimoda, Shinri Oda, Kaori Hoshikawa, Takahiro Osada, Rie Aoki, Azusa Sunaga. Hyperintense posterior cerebral artery sign in patients with reversible cerebral vasoconstriction syndrome. 16-Nov-2021;12:558. Available from: https://surgicalneurologyint.com/surgicalint-articles/11231/
Abstract
Background: This study investigated hyperintense vessel signs (HVS) on fluid-attenuated inversion recovery imaging in the P1–2 portions of posterior cerebral arteries (PCAs) as a “hyperintense PCA sign” and HVS of cortical arteries. We retrospectively examined whether these signs would be useful in diagnosing reversible cerebral vasoconstriction syndrome (RCVS) in the acute phase.
Methods: Eighty patients with RCVS who underwent initial magnetic resonance imaging (MRI) within 7 days of onset were included in this study. HVS and related clinical factors were examined.
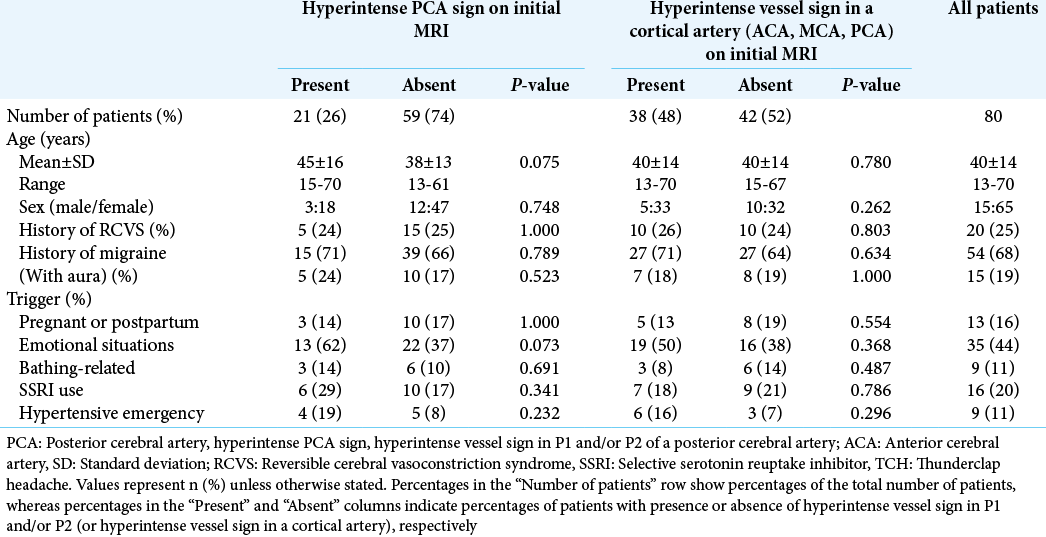
Results: On initial MRI of RCVS patients, hyperintense PCA sign and HVS of cortical arteries were seen in 21 cases (26%) and 38 cases (48%), respectively. In patients showing hyperintense PCA sign, vasoconstriction of the A2–3 portion was a significant clinical factor. Conversely, vasoconstriction of the M1 and P1 portions and the presence of white matter hyperintensity on initial and chronic-stage MRI were significantly associated with the presence of HVS in cortical arteries.
Conclusion: Because rich collateral flow exists around PCAs, the frequency of hyperintense PCA sign is not high. However, hyperintense PCA sign findings in patients with suspected RCVS offer credible evidence of extreme flow decreases due to vasoconstriction in peripheral PCAs and other arteries associated with the collateral circulation of PCAs. Conversely, HVS in cortical arteries tend to reflect slow antegrade circulation due to vasoconstriction of peripheral vessel and major trunks. Both signs appear useful for auxiliary diagnosis of acute-phase RCVS.
Keywords: Fluid-attenuated inversion recovery, Hyperintense vessel sign, Posterior cerebral artery, Reversible cerebral vasoconstriction syndrome
INTRODUCTION
“Hyperintense vessel signs” (HVS) or “intra-arterial high signals” are seen when occlusion of a blood vessel is depicted as high signals on fluid-attenuated inversion recovery (FLAIR) imaging.[
In the past, HVS on FLAIR images have been reported as a neuroradiological finding in the acute phase of RCVS.[
At present, as vasoconstriction vessels are located peripherally during the acute phase,[
MATERIALS AND METHODS
Normal control group
The normal control group comprised 166 patients who visited our hospital with neurological conditions other than cerebrovascular disorders, brain tumor, demyelinating diseases, dementia, or metabolic diseases, and who underwent MRI of the head that showed no abnormalities (50 men, 116 women; mean age, 54 ± 16 years). Normal MRI findings were defined as a lack of arteriosclerotic stenosis of major intracranial arteries (including PCAs), asymptomatic or symptomatic cerebral infarction, cerebral hemorrhage including microbleeds, or hydrocephalus. Regarding periventricular and deep and subcortical white matter hyperintensity (DSWMH), grades 0–1 of the Fazekas scale[
Patient population
Patients diagnosed with RCVS in our institution according to our previously reported criteria for RCVS[
Our database included 102 RCVS patients between October 2010 and November 2020. We excluded 22 RCVS patients who visited our hospital in the subacute or chronic phase and who were therefore not examined by initial MRA or MRI within 7 days of RCVS onset. The remaining 80 RCVS patients who underwent initial MRI within 7 days of RCVS onset were included in this retrospective study. As much as possible, we performed sequential MRA during the period from onset to remission of TCH and within 14 days after TCH remission. No significant differences in demographic variables were present between enrolled and excluded patients.
Imaging protocol
The distinction between RCVS and primary angiitis of the central nervous system was diagnosed with reference to the RCVS2 score.[
Definitions of variables
TCH was defined as a severe pain peaking within seconds. The presence of TCH was diagnosed by a thorough interview of the patient. TCH remission was defined as “the time at which the last TCH improved.” Hypertensive emergency was defined as systolic blood pressure over 180 mmHg or diastolic blood pressure over 120 mmHg.
White matter hyperintensity (WMH) was defined as hyperintensity on FLAIR imaging without hypointensity on T1WI. Progression of WMH after RCVS was assessed by comparison of MRI at onset and 3 months after onset. We assessed progression of periventricular WMH using the Fazekas scale,[
We divided RCVS patients into two groups depending on the presence or absence of HPS. Furthermore, we divided RCVS patients into two groups depending on the presence or absence of HVS in a cortical artery, and we investigated differences in clinical features. HVS was defined as focal, tubular, or serpentine hyperintensities in the subarachnoid space, relative to those in the cerebrospinal fluid and corresponding with the typical arterial course.[
Figure 1:
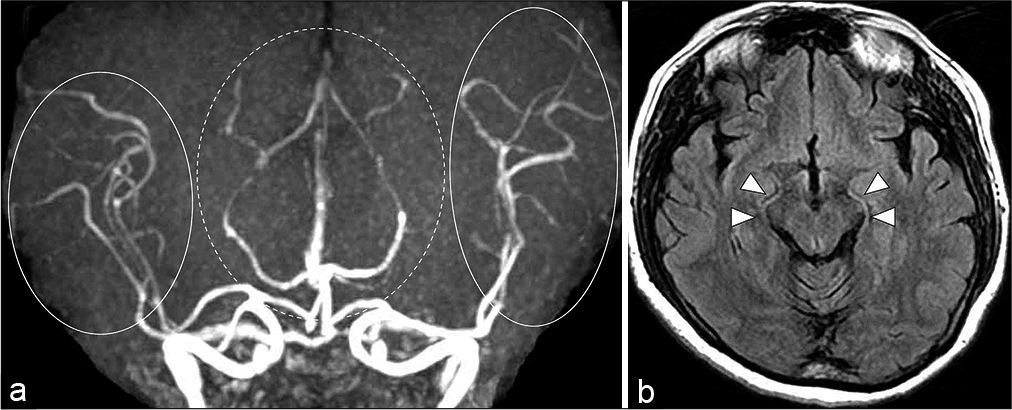
Images from a 55-year-old woman with reversible cerebral vasoconstriction syndrome. (a) The initial magnetic resonance angiography obtained 8 h after onset of thunderclap headache shows vasoconstrictions in bilateral P2–3 portions of the posterior cerebral arteries (dotted circle), and bilateral M2–3 portions of the middle cerebral artery (circle). (b) Fluid-attenuated inversion recovery obtained at the same time as magnetic resonance angiography shows bilateral hyperintense posterior cerebral arteries signs in the crural and ambient cisterns (white arrowheads).
Figure 2:
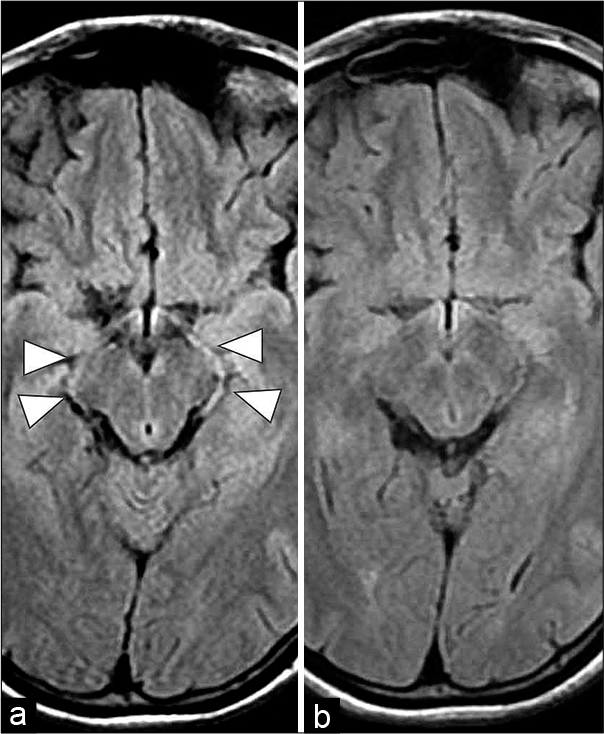
Images from a 17-year-old woman with reversible cerebral vasoconstriction syndrome. (a) Fluid-attenuated inversion recovery obtained 4 days after onset of Thunderclap headache shows bilateral hyperintense posterior cerebral arteries signs (HPSs) in the crural and ambient cisterns (white arrowheads). (b) In the chronic phase of fluid-attenuated inversion recovery, findings of HPS have disappeared.
Figure 3:
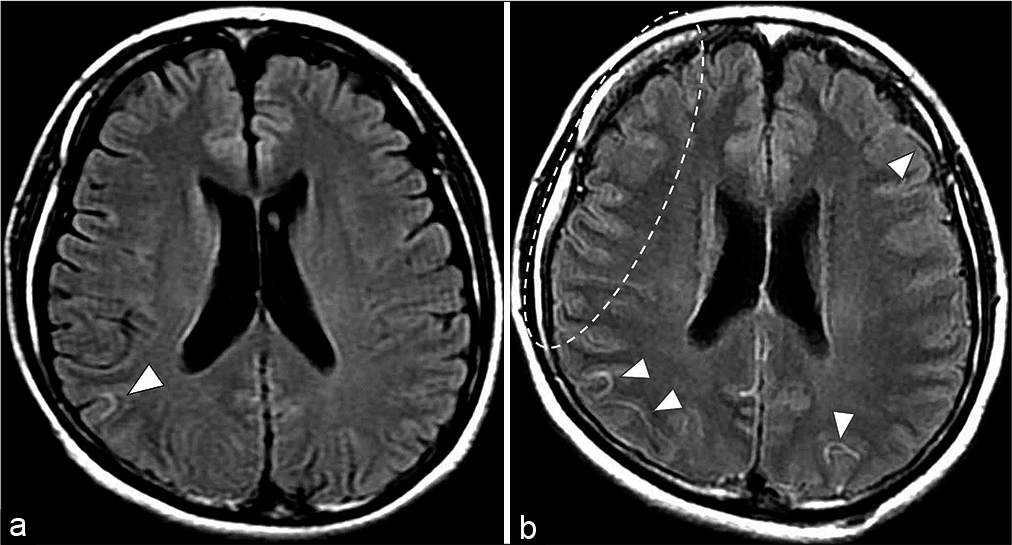
Images from a 31-year-old woman with reversible cerebral vasoconstriction syndrome. (a) Initial fluid-attenuated inversion recovery obtained 2 days after onset shows Hyperintense vessel signs in the right cortical artery (white arrowheads). (b) At 11 days after onset, centripetal propagation of vasoconstriction in bilateral P1–2 portions of the posterior cerebral arteries, left M1 portions, basilar artery, and right vertebral artery are apparent on follow-up magnetic resonance angiography. Fluid-attenuated inversion recovery obtained at the same time as magnetic resonance angiography shows multiple hyperintense vessel signs of the cortical artery (white arrowheads and dotted circle).
MRI findings were interpreted by at least two senior stroke neurosurgeons (M.S. and S.O., with 38 and 33 years of experience, respectively). When neurosurgeons disagreed about findings, they consulted with each other to reach a consensus decision. Outcomes were assessed at 3–6 months after onset using modified Rankin scale scores.
Treatment protocol
Vasoactive medications such as triptans were stopped, and symptomatic analgesic treatment was used in all patients without a standard protocol. Oral administration of cilostazol or lomerizine hydrochloride was recommended for the prevention of cerebral vasoconstriction. Administration of steroids was avoided. For patients with severe TCH, low-dose propofol (30–50 mg/h) was infused intravenously. For five of the ten patients who experienced a hypertensive emergency, nicardipine was used with intravenous infusion of the dose adapted to normalize blood pressure levels.
Institutional review board approval
Study approval was obtained from the Institutional Review Board for Clinical Research (approval no. 21R-037) and Conflict of Interest Management Committee (approval no. 21-037) at our university. We performed MRI after obtaining oral informed consent from each patient.
Statistical analysis
In patients with RCVS, the significance of clinical factors potentially associated with HPS and HVS in a cortical artery was determined by the two-tailed Fisher’s exact test. Continuous variables (age and timing of initial MRI after onset) were tested using an independent sample two-tailed Student’s t-test. Clinical factors showing a significance level of P < 0.10 were entered into multivariate logistic regression analysis with the presence of HPS and HVS in a cortical artery as the dependent variable. All statistical analyses were performed using commercially available software (Statistical Package for the Social Sciences [SPSS] for Windows version 22.0; Mehta and Patel/SPSS, Chicago, IL).
RESULTS
Incidence of HPS in the normal control group
Among the 166 patients in the normal control group (without asymptomatic arteriosclerotic stenosis of the PCA), the prevalence of HPS was 2% (3/166).
Pre-onset clinical features and HVS
[
The sensitivity and specificity of HPS on initial MRI as a diagnostic method (or “marker?”) for RCVS were 26% (21/80) and 98% (163/166), respectively, with reference to the normal control group. In RCVS patients with findings of HPS and HVS for a cortical artery, no relevant pre-onset clinical factors were found [
Findings of MRI and post-onset clinical factors
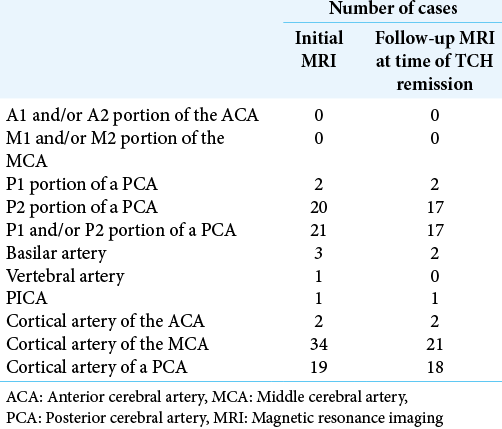
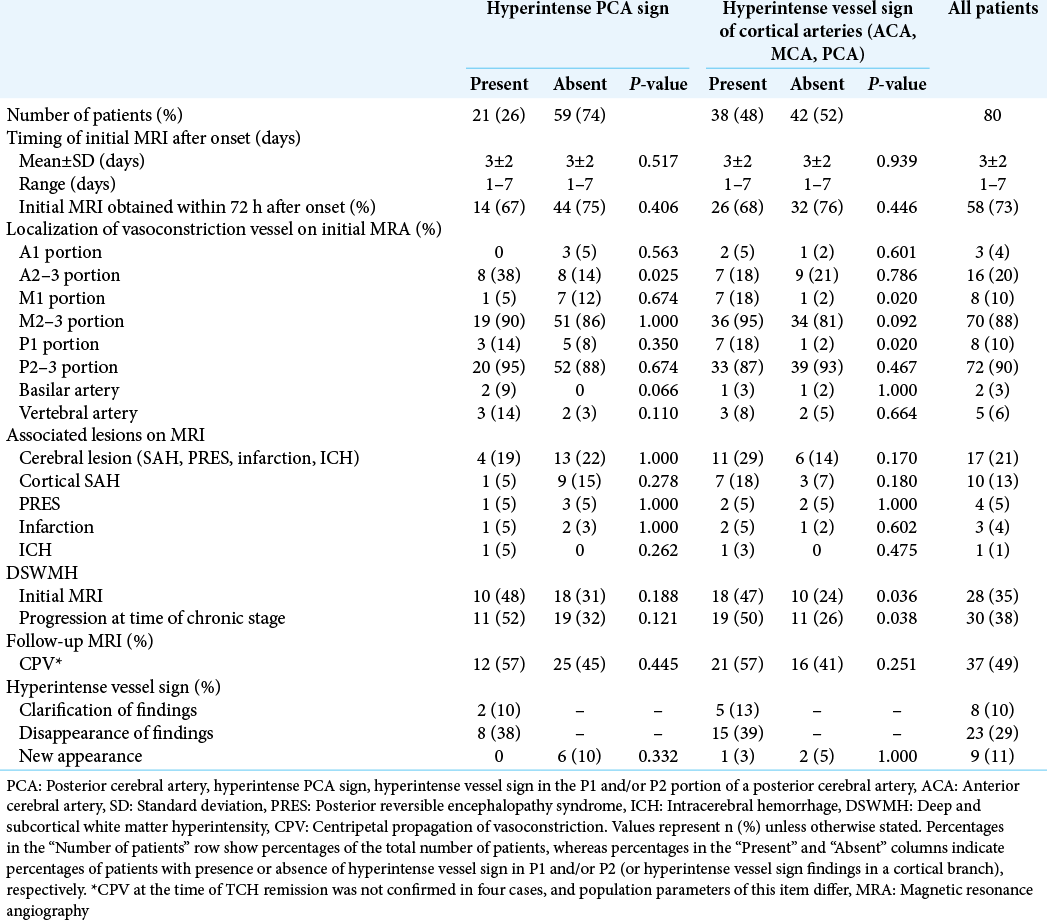
Among all patients, 73% (58 of 80 cases) underwent MRI during the acute phase within 72 h of onset. Among the localizations of vasoconstriction, vasoconstriction of A2–3 was significantly associated with the presence of HPS [
In RCVS patients with findings of HVS in a cortical artery, timing of initial MRI after onset and CPV showed no significant association [
After 3–6 months, modified Rankin scale score was 0 in all 80 of these RCVS patients, and all were able to resume prior daily activities.
Changes to findings of HPS and HVS in a cortical artery on follow-up MRI
On follow-up MRI at the time of TCH remission, the frequency of clarification of findings and new findings of HPS and HVS in a cortical artery was low, at about 10% [
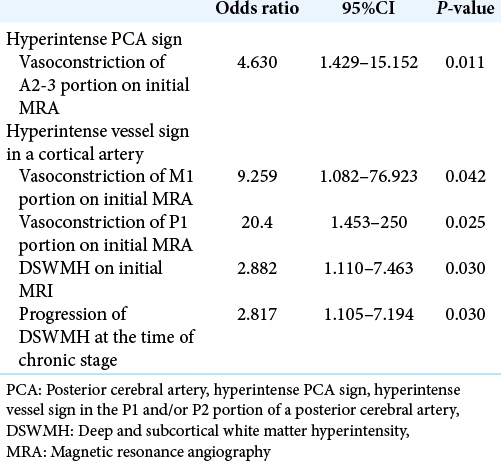
Clinical factors related to HPS and HVS in a cortical artery on multivariate analysis
Multivariate step-wise logistic regression analysis revealed that the presence of vasoconstriction in the A2–3 portion was significantly associated with the presence of HPS (P = 0.011) [
Inter-reader reproducibility
Inter-reader agreement was excellent for both HPS (97.5% agreement, κ = 0.935 (95% confidence interval [CI] 0.780–0.982) and HVS of cortical arteries (93.8% agreement, κ = 0.875 (95%CI 0.724–0.942)) on initial MRI in RCVS patients.
DISCUSSION
Mechanism and clinical significance of HPS findings in RCVS
While the mechanisms underlying HVS remain to be established, stationary blood and slow antegrade or retrograde collateral circulation at a site peripheral to arterial occlusion or severe stenosis have been suggested as possible explanations for HVS.[
Since severe vasoconstriction of the ICA is rarely associated with patients with RCVS, obtaining HVS findings in M1 and A1 seemed rare in this study.
On the other hand, when performing endovascular surgery for aneurysms of a PCA, the low incidence of complications due to parent artery occlusion is related to the rich anastomotic collaterals that exist between the area of the PCA and the areas of other arteries, which include: (1) collateral circulation between the lateral posterior choroidal artery (branch of the P2 segment) and the anterior choroidal arteries (branches of the ICA); (2) collateral circulation between the long circumflex arteries (branches of the P1 segment) and the superior cerebellar artery territory at the level of the quadrigeminal plate; (3) collateral circulation between the splenial artery (branch of the P3–P4 segments) and posterior pericallosal artery (branch of the ACA); and (4) collateral circulation between the inferior temporal branches of PCAs and the superior temporal branches of the MCA.[
Therefore, due to the usual presence of rich collateral flows around PCAs, HPS rarely appears because blood flow through the proximal PCA (P1–2) would be expected to be maintained through these rich collaterals even if peripheral PCA flow is decrease. Furthermore, this study found no significant association between the development of HPS and vasoconstriction of the basilar artery or vertebral artery as arteries proximal to the PCA. In addition, vasoconstriction of the P1 and P2–3 portions was not significantly associated with the development of HPS. We, therefore, speculated that HPS may be caused by the coexistence of not only decreased flow due to vasoconstriction in PCA peripherals, but also decreased flow due to vasoconstriction of multiple other arteries associated with the collateral circulation of the PCAs. Because HPS comprehensively evaluate flow decreases due to vasoconstriction of not only PCAs, but also multiple vessels associated with the network of collateral circulation of the PCAs, we think that HPS represent a neuroradiological finding suitable for diagnosing RCVS in the acute phase. We believe that absence of HPS is unsuitable for excluding the diagnosis due to the relatively low frequency of this sign (low sensitivity), but their presence is extremely useful for definitive diagnosis of RCVS. Because vasoconstriction vessels are located peripherally during the acute phase,[
In this study, the vasoconstriction that occurred in A2–3, which was associated with the collateral circulation of PCAs, was significantly associated with the development of HPS. Although M2–3 and P2–3 are similarly associated with the collateral circulations of the PCA and PCAs, no significant association was evident between M2–3 and P2–3 vasoconstriction and HPS findings. In this study, the incidences of M2–3 and P2–3 vasoconstrictions were as high as ≥85% with or without findings of HPS. We, therefore, speculate that whether M2–3 and P2–3 vasoconstriction results in HPS depends on individual differences in the degree or location of the collateral circulation associated with the PCAs.
Besides RCVS, other disorders that may show HPS findings include PCA atherosclerosis. This can be easily distinguished from RCVS by the presence or absence of TCH as a symptom and vasoconstrictions of multiple other arteries.
Clinical factors related to HVS in a cortical artery
In our study, MCA was the most frequent site of HVS of cortical arteries, but the cause remains unknown. One possible reason is that HVS of the insular-opercular segment of the MCA around the sylvian fissure are easily identified. In this study, because M1 and P1 are vessels proximal to the cortical artery, vasoconstrictions of the M1 and P1 were significantly associated with findings of HVS in a cortical artery on initial MRI of RCVS patients. HVS in a cortical artery also correlated significantly with the presence of DSWMH on initial MRI, and progression of DSWHM on MRI in the chronic phase. We interpret DSWMH on the initial MRI as a lesion caused by microcirculatory dysfunction due to some previous cerebral small vessel disease.[
Changes to findings of HPS and HVS in a cortical artery
We reported that, from findings of sequential MRA before and after TCH remission, CPV gradually progresses after the onset of RCVS, peaks at the time of TCH remission, and does not progress further upon TCH remission.[
Limitations
In this study, MRI findings were interpreted by senior stroke neurosurgeons, but blinding to the timing of imaging was not performed. Because nimodipine has not been approved for use in Japan as a calcium channel antagonist for preventing vasoconstriction, we could not administer this drug. Our results thus may not be generalizable to hospital facilities using nimodipine. This was a retrospective study of a small group of patients, and prospective studies with a greater number of cases are necessary in the future.
CONCLUSION
Because rich collateral flows exist around the PCAs, HPS rarely appear; that is, flow through proximal PCAs would be expected to be maintained through the rich collateral vasculature even if peripheral PCA flow is decreased. An absence of HPS is therefore unsuitable for excluding the diagnosis of RCVS. However, HPS findings obtained from patients with suspected RCVS offer clinicians credible evidence of extreme flow decreases due to vasoconstriction in peripheral PCAs and other arteries associated with the collateral circulation of PCAs. Conversely, HVS in a cortical artery tend to reflect slow antegrade circulation due to vasoconstriction of peripheral vessels and major trunks and were significantly associated with progression of DSWMH in the chronic phase of RCVS. Although the diagnosis of RCVS is frequently missed because the vasoconstriction vessels are located peripherally during the acute phase, we emphasize that both HPS and HVS in a cortical artery are extremely useful when clinicians encounter cases in which they are confused about the acute diagnosis of RCVS.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Chen SP, Fuh JL, Lirng JF, Wang SJ. Hyperintense vessels on flair imaging in reversible cerebral vasoconstriction syndrome. Cephalalgia. 2012. 32: 271-8
2. Chen SP, Fuh JL, Wang SJ. Reversible cerebral vasoconstriction syndrome: Current and future perspectives. Expert Rev Neurother. 2011. 11: 1265-76
3. Chen SP, Fuh JL, Wang SJ, Chang FC, Lirng JF, Fang YC. Magnetic resonance angiography in reversible cerebral vasoconstriction syndromes. Ann Neurol. 2010. 67: 648-56
4. Ciceri EF, Klucznik RP, Grossman RG, Rose JE, Mawad ME. Aneurysms of the posterior cerebral artery: Classification and endovascular treatment. Am J Neuroradiol. 2001. 22: 27-34
5. Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007. 130: 3091-101
6. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am J Roentgenol. 1987. 149: 351-6
7. Kameda T, Namekawa M, Shimazaki H, Minakata D, Matsuura T, Nakano I. Unique combination of hyperintense vessel sign on initial FLAIR and delayed vasoconstriction on MRA in reversible cerebral vasoconstriction syndrome: A case report. Cephalalgia. 2014. 34: 1093-6
8. Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: Evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab. 1998. 18: 570-9
9. Krings T, Noelchen D, Mull M, Willmes K, Meister IG, Reinacher P. The hyperdense posterior cerebral artery sign: A computed tomography marker of acute ischemia in the posterior cerebral artery territory. Stroke. 2006. 37: 399-403
10. Lee KY, Latour LL, Luby M, Hsia AW, Merino JG, Warach S. Distal hyperintense vessels on FLAIR: An MRI marker for collateral circulation in acute stroke?. Neurology. 2009. 72: 1134-9
11. Legrand L, Tisserand M, Turc G, Naggara O, Edjlali M, Mellerio C. Do FLAIR vascular hyperintensities beyond the DWI lesion represent the ischemic penumbra?. Am J Neuroradiol. 2015. 36: 269-74
12. Noguchi K, Ogawa T, Inugami A, Fujita H, Hatazawa J, Shimosegawa E. MRI of acute cerebral infarction: A comparison of FLAIR and T2-weighted fast spin-echo imaging. Neuroradiology. 1997. 39: 406-10
13. Omura-Matsuoka E, Yagita Y, Sasaki T, Terasaki Y, Oyama N, Sugiyama Y. Hypertension impairs leptomeningeal collateral growth after common carotid artery occlusion: Restoration by antihypertensive treatment. J Neurosci Res. 2011. 89: 108-16
14. Pantoni L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010. 9: 689-701
15. Rocha EA, Topcuoglu MA, Silva GS, Singhal AB. RCVS2 score and diagnostic approach for reversible cerebral vasoconstriction syndrome. Neurology. 2019. 92: e639-47
16. Sanossian N, Saver JL, Alger JR, Kim D, Duckwiler GR, Jahan R. Angiography reveals that fluid-attenuated inversion recovery vascular hyperintensities are due to slow flow, not thrombus. Am J Neuroradiol. 2009. 30: 564-8
17. Shimoda M, Oda S, Hirayama A, Imai M, Komatsu F, Hoshikawa K. Centripetal propagation of vasoconstriction at the time of headache resolution in patients with reversible cerebral vasoconstriction syndrome. Am J Neuroradiol. 2016. 37: 1594-8
18. Shimoda M, Oda S, Shigematsu H, Hoshikawa K, Imai M, Komatsu F. Clinical significance of centripetal propagation of vasoconstriction in patients with reversible cerebral vasoconstriction syndrome: A retrospective case-control study. Cephalalgia. 2018. 38: 1864-75
19. Zhang Y, Parikh A, Qian S. Migraine and stroke. Stroke Vasc Neurol. 2017. 2: 160-7