- Department of Neurology, The Neurology Group, Pomona, United States.
- Department of Medicine, Western University of Health Sciences, Pomona, United States.
- Department of Biology, University of California, Irvine, California, United States.
- Department of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States.
- Department of Biology, University of Arizona, Tucson, Arizona, United States.
Correspondence Address:
Sara Zarei, Department of Neurology, The Neurology Group, Pomona, California, United States.
DOI:10.25259/SNI_670_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sara Zarei1, Setareh Kamali2, William Narinyan2, Farnoush Nasouri3, Sara Hassani4, Abdul Mahmoud Ibrahim1, Rojeen Zarei5, Sadiq Altamimi1. Idiopathic intracranial hypertension associated with polycystic ovarian syndrome, sensorineural hearing loss, and elevated inflammatory markers that lead to bilateral blindness: A case report with literature review. 17-Nov-2023;14:399
How to cite this URL: Sara Zarei1, Setareh Kamali2, William Narinyan2, Farnoush Nasouri3, Sara Hassani4, Abdul Mahmoud Ibrahim1, Rojeen Zarei5, Sadiq Altamimi1. Idiopathic intracranial hypertension associated with polycystic ovarian syndrome, sensorineural hearing loss, and elevated inflammatory markers that lead to bilateral blindness: A case report with literature review. 17-Nov-2023;14:399. Available from: https://surgicalneurologyint.com/surgicalint-articles/12641/
Abstract
Background: Pseudotumor cerebri (PTC) or idiopathic intracranial hypertension (IIH) is characterized by elevated intracranial pressure without hydrocephalus or mass lesion, with normal cerebrospinal fluid (CSF) studies and neuroimaging. The exact cause remains uncertain, but potential mechanisms include increased CSF production, impaired CSF absorption, cerebral edema, and abnormal cerebral venous pressure gradients. Patients may present with various accompanying symptoms such as unilateral or bilateral visual obscuration, pulsatile tinnitus, back pain, dizziness, neck pain, blurred vision, cognitive difficulties, radicular pain, and typically intermittent horizontal diplopia.
Case Description: We report a case of a 32-year-old female who initially presented with chronic headaches and oligomenorrhea, which resulted in the diagnosis of polycystic ovary syndrome (PCOS) a few years before the initial diagnosis of PTC. Despite receiving maximum medical treatment and undergoing optic nerve sheath fenestration, the patient experienced complete bilateral vision loss. Nearly 5 years later, the patient sought care at our outpatient neurology clinic, presenting with symptoms including tinnitus, left-sided hearing loss, and joint pain with elevated inflammatory markers and headaches. The focus of this research was to discuss the pathophysiology of each of these comorbidities.
Conclusion: This case report aims to explore the pathophysiological relationships between PTC and concurrent comorbidities, including PCOS, sensorineural hearing loss, empty sella (ES) syndrome, and elevated inflammatory markers. Remarkably, no other PTC case with this unique constellation of concurrent comorbidities have been reported in existing medical literature. The case report underscores the critical importance of early diagnosis of IIH and prompt medical intervention, particularly in patients with PCOS experiencing chronic headaches.
Keywords: Bilateral blindness, Empty sella syndrome, Idiopathic intracranial hypertension, Polycystic ovary syndrome, Sensorineural hearing loss, Pseudotumor cerebri
INTRODUCTION
In this case report, we will study the association of idiopathic intracranial hypertension (IIH), polycystic ovary syndrome (PCOS), sensorineural hearing loss, and elevated inflammatory markers, in a patient who, despite receiving optimal medical treatment and undergoing optic nerve sheath fenestration (ONSF), unfortunately, experienced bilateral blindness. The focus of the case report will be to delve into the pathophysiology of each of these comorbidities and explore how they can manifest and interact differently in individual patients. Emphasizing the importance of early diagnosis, particularly in patients with PCOS who experience chronic headaches, this research underscores the significance of prompt treatment and the need to investigate the underlying mechanisms.
Pseudotumor cerebri (PTC) also known as IIH is primarily associated with raised intracranial pressure with no hydrocephalus or mass lesion, in the presence of normal cerebrospinal fluid (CSF) studies and neuroimaging, without any evident underlying cause.[
PTC commonly affects women aged 20–44, with a female-to-male ratio of 8:1, with a yearly incidence of 19.3/100,000 within the United States, especially among obese women of child-bearing age who weigh more than 20% of their ideal body weight.[
The exact underlying cause of IIH remains uncertain, but various mechanisms have been suggested to elucidate the elevated CSF pressure. These mechanisms include increased production of CSF, impaired absorption of CSF at arachnoid granulations or lymphatics, cerebral edema, and elevated cerebral venous pressure resulting from abnormal venous pressure gradients. These factors have been proposed as potential contributors to the raised CSF pressure in IIH.[
The majority of individuals with IIH commonly experience a headache that progressively becomes more severe and frequent, as outlined in the International Classification of Headache Disorders, 3rd edition. This headache phenotype can vary significantly and may resemble other primary headache disorders. In addition, patients may present with various accompanying symptoms such as unilateral or bilateral visual obscuration, pulsatile tinnitus, back pain, dizziness, neck pain, blurred vision, cognitive difficulties, radicular pain, and typically intermittent horizontal diplopia. It is important to note that none of these symptoms are specific to IIH and cannot definitively indicate its presence. The investigation and management of IIH rely on the assessment of individual symptoms and signs, necessitating a collaborative approach involving experts from multiple disciplines.[
The potential of neuroimaging signs of elevated intracranial pressure is often underutilized in the diagnostic evaluation of suspected PTC. Studies have found that flattening of the posterior sclera emerged as the most sensitive indicator of elevated intracranial pressure in patients diagnosed with PTC.[
Diagnosis of PTC is based on the modified Dandy criteria and varies based on the presence or absence of papilledema. Required criteria of PTC in patients with papilledema include normal neurologic examination except for cranial nerve abnormalities, normal brain parenchyma without evidence of hydrocephalus, mass, or structural lesion on MRI neuroimaging with and without gadolinium contrast, normal CSF composition, and an elevated lumbar puncture opening pressure >250 mm in adults or 280 mm in children.[
Conservative management for PTC begins with identifying and targeting known risk factors. Therefore, regardless of disease severity, initiating a weight loss program has been shown to improve the disease course.[
The main objective of this case is to delve into the pathophysiological associations between PTC and concurrent comorbidities, including PCOS, sensorineural hearing loss, ES syndrome, and elevated inflammatory markers. In the current medical literature, there is no documentation of another PTC case presenting the constellation of concurrent comorbidities observed in this patient. The manuscript underscores the importance of early diagnosis and prompt medical management, particularly in patients with PCOS who experience chronic headaches. It highlights the significance of recognizing and addressing IIH and its associated comorbidities to prevent potential complications and optimize patient outcomes.
CASE DESCRIPTION
A 32-year-old female with a medical history of PTC presented to the outpatient neurology clinic complaining of worsening headache, joint pain, hearing loss in the left ear, and tinnitus. She had previously been diagnosed with PTC and her symptoms were progressively increasing for 5 years. Family history and psychosocial history were unremarkable. Initially, the patient was on acetazolamide but showed no improvement. The patient has had a lumbar puncture that led to the relief of her symptoms for only a few days. A VP shunt (VPS) placement surgery could not be performed due to the patient having an implantable cardioverter defibrillator (ICD) for cardiomyopathy a few years earlier.
On physical examination, the patient was found to be morbidly obese with a body mass index (BMI) of 64.5. Eye examination revealed mildly dilated pupils bilaterally with significant loss of vision in both eyes, with only minimal perception of hand waving. The patient had light perception but exhibited an afferent pupillary defect and abducens nerve palsy. A fundoscopic examination revealed gross papilledema bilaterally with optic disc pallor that indicated optic nerve atrophy. The otoscopic examination was normal and Weber and Rinne tests suggested left-sided sensorineural hearing loss.
The patient’s remaining neurological examinations such as the remainder of cranial nerves, motor and sensory functions, reflexes, and mental status were unremarkable.
A review of the patient’s medical record revealed that the patient had also received ONSF which entails creating an opening or window in the optic nerve sheath to drain CSF from the subarachnoid space surrounding the optic nerves for decompression.
According to the patient, optic nerve fenestration initially was helpful, but later she became legally blind despite the maximum medical management and even after optic nerve fenestration. Subsequent investigation revealed that the patient initially presented with chronic headaches and oligomenorrhea, which led to the diagnosis of polycystic ovarian syndrome (PCOS) years before her initial diagnosis of PTC [
Figure 1:
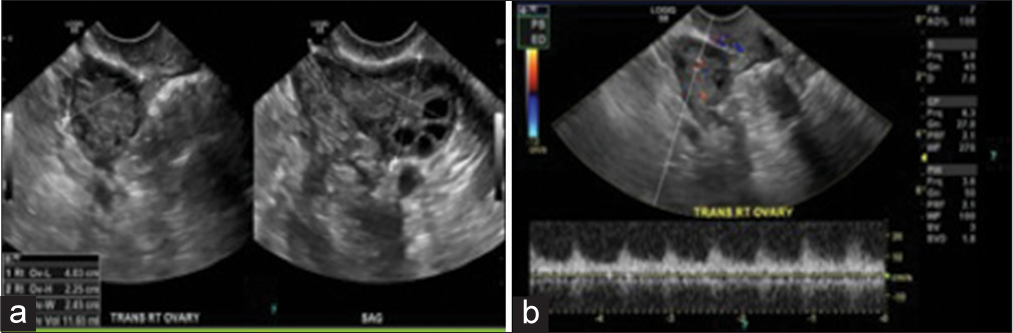
(a) Transvaginal ultrasound of the right ovary in grayscale view demonstrates the morphological features of the polycystic ovarian disease: Hyperechoic central stroma, peripheral location of follicles (a string of pearls sign), follicles of similar size measuring 2–9 mm, and increased right ovary size and volume; the right ovary measures approximately 40 mm × 22 mm × 24 mm (length × width × thickness) (corresponding to a volume of 11.65 cm3). An ovary typically weighs 2–8 g. However, they change during life and double in size during pregnancy. (b) Transvaginal ultrasound of the right ovary in color Doppler study demonstrates the mean spectral of the ovarian stromal arteries. However, using color Doppler transvaginal ultrasonography in the clinical diagnosis of patients with polycystic ovary syndrome is not beneficial.
Figure 2:
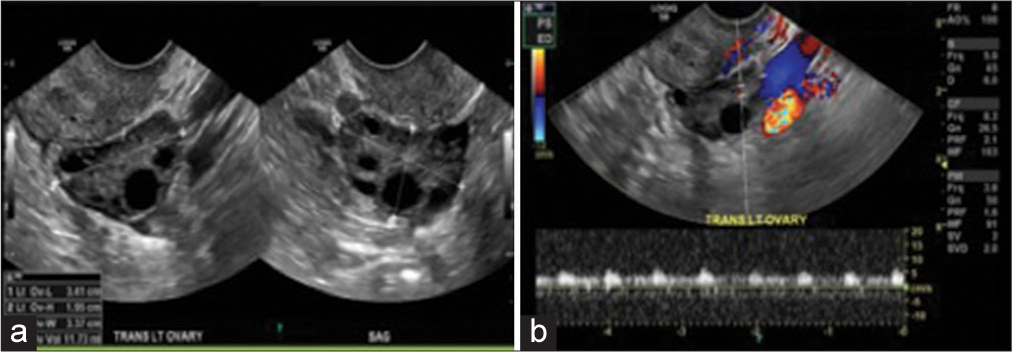
(a) Transvaginal ultrasound of the left ovary in grayscale view demonstrates the morphological features of polycystic ovarian disease: Hyperechoic central stroma, peripheral location of follicles (a string of pearls sign), follicles of similar size measuring 2–9 mm, and increased left ovary size and volume; the left ovary measures approximately 34 mm × 19 mm × 33 mm (length × width × thickness) (corresponding to a volume of 11.73 cm3). An ovary typically weighs 2–8 g. However, they change during life and double in size during pregnancy, (b) transvaginal ultrasound of the left ovary in color Doppler study demonstrates the mean spectral of the ovarian stromal arteries. However, using color Doppler transvaginal ultrasonography in the clinical diagnosis of patients with polycystic ovary syndrome is not beneficial.
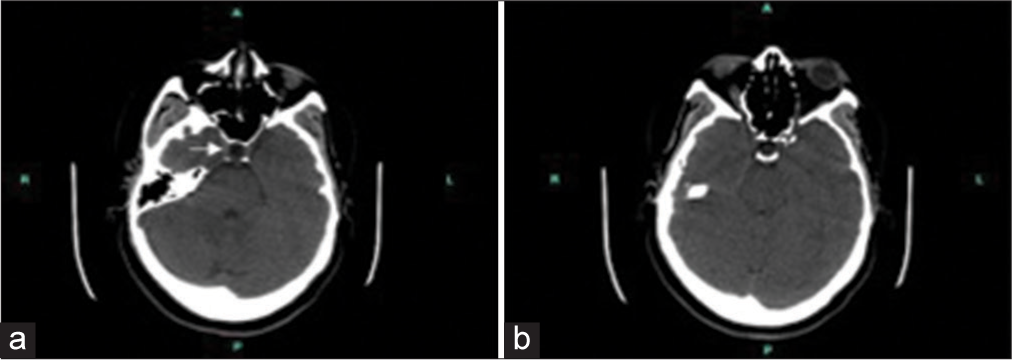
During the follow-up visit at our outpatient neurology clinic, visual-evoked potential showed normal latencies of bilateral optic nerves. Brain auditory-evoked response test revealed left peripheral acoustic nerve neuropathy. The patient’s laboratory result showed rheumatoid factor: 119 IU/mL (normal reference 0.0–13.9) and a high level of C-reactive protein: 13.2 mg/L (normal reference 0.0–4.9); negative Sjogren antibodies (SS-A, SS-B); and erythrocyte sedimentation rate was within normal range. The patient reported experiencing joint pain and stiffness, leading to a referral to a rheumatologist for further evaluation. She was also ordered a non-contrast brain computed tomography (CT) scan to exclude any space-occupying lesions. CT scan findings showed a 3 × 5 mm fat density structure in the third ventricular roof region beneath the body of the corpus callosum and an empty appearance of the sella turcica [
The patient was started on Topiramate 25 mg oral tablets. During the 3-month follow-up visit, the patient’s condition did not show any improvement. To address this, the dosage of topiramate was increased to 100 mg, while closely monitoring for any potential side effects and she was also started on Furosemide 80 mg. At the subsequent visit, there was still no improvement observed. The patient still complained of headaches and tinnitus. As a result, the dosage of topiramate was further increased to 150 mg, which provided some relief from headaches for a short period of time. To address the ongoing symptoms, the medication regimen was adjusted once again. Promethazine HCL 25 mg oral tablets and Diamox Sequel 500 mg oral extended-release were incorporated into the treatment plan. After a period of 3 months, the dosage of Diamox was raised to 1000 mg, and careful monitoring for potential side effects was implemented. In addition, Phenergan 25 mg was continued alongside the adjusted medication plan. The patient stayed on the regimen for over 6 months and is still being followed up.
DISCUSSION
This case report describes a 32-year-old female who initially presented with chronic headaches and oligomenorrhea, which resulted in the diagnosis of PCOS a few years before the initial diagnosis of PTC. Despite receiving maximum medical treatment and undergoing ONSF, the patient experienced complete bilateral vision loss. Nearly 5 years later, the patient sought care at our outpatient neurology clinic, presenting with symptoms including tinnitus, left-sided hearing loss, and joint pain with elevated inflammatory markers and headaches.
Hence, in this case report, we will explore the pathophysiological connections between PTC and its concurrent comorbidities, which encompass PCOS, sensorineural hearing loss, ES syndrome, and elevated inflammatory markers. In the existing medical literature, there is no documented instance of another PTC case manifesting this particular combination of concurrent comorbidities. This case report underscores the critical importance of early diagnosis and expeditious medical intervention, especially in patients with PCOS who are afflicted by chronic headaches.
Despite receiving optimal medical treatment and undergoing ONSF, this patient unfortunately experienced legal blindness. ONSF is generally advised for individuals presenting with visual symptoms, whereas shunting procedures are typically reserved for those with headaches.[
In most patients, prompt management of PTC results in a good prognosis with a rapid improvement of symptoms after conservative medical management including diuretics and acetazolamide.[
Our patient also exhibited symptoms related to ES syndrome. ES is characterized by the filling of the sella turcica with CSF, resulting in the compression of pituitary tissue until it lines the floor and walls of the sella. Primary ES refers to the entry of CSF into the sella through a defect in the sellar diaphragm, which could be linked to elevated intracranial pressure.
When comparing PTC patients to a control population, it was found that the sagittal cross-sectional area of the sella turcica was increased by an average of 38% in patients with PTC compared to the control group.[
As previously mentioned, this patient was diagnosed with PCOS a few years before her diagnosis of IIH. The study conducted by Cosar et al. observed an incidence of three cases of IIH per 30 women with PCOS and headache. The previous studies reported a prevalence of well-documented PCOS in women referred for IIH ranging from 39% to 57%. These rates were significantly higher, 5–8 times greater, than the 4–10% prevalence of PCOS in the general unselected population.[
Hence given the association between PCOS, obesity, and the development of IIH, it is crucial to carefully evaluate and monitor patients with PCOS who present with chronic headaches, especially when accompanied by ES. Prompt diagnosis and management of IIH are important to prevent potential complications and optimize patient outcomes.
This patient also exhibited left-sided sensorineural hearing loss. It is widely recognized that the visual impairments observed in IIH are primarily attributed to the compression of the optic nerve. The increased pressure within the intracranial space transmits to the optic nerve sheath through the optic canal, obstructing the axoplasmic transmission in the optic nerve axons. Consequently, this leads to swelling of the optic nerve fibers. Similarly, it is postulated that the audio-vestibular symptoms experienced by individuals with IIH result from the compression and subsequent edema of the vestibulocochlear nerve due to elevated intracranial pressure.[
Furthermore, this patient presented with elevated inflammatory markers and experienced joint pains, necessitating a referral to a rheumatologist for further evaluation and management.
Considering the patient’s elevated BMI value, it is important to acknowledge that obesity plays a significant role in triggering a chronic proinflammatory state within the body. This state of inflammation has been strongly associated with the development of IIH. The association between obesity and the development of IIH may be partly explained by proposed mechanisms involving inflammatory processes. One such mechanism involves the dysregulation of the enzyme 11b-hydroxysteroid dehydrogenase type 1 (11b-HSD1), which plays a role in modulating glucocorticoids and regulating CSF secretion. Increased activity of 11b-HSD1 has been observed in both obesity and IIH, potentially leading to elevated local cortisol levels. Prolonged exposure to high cortisol levels can stimulate the production of proinflammatory mediators and potentially impact CSF production. Studies have shown that weight loss and reduced intracranial pressure in IIH patients are associated with decreased levels of 11b-HSD1.[
The management of our patient’s symptoms predominantly focused on individualized adjustments to medication types and dosages. Given the complex nature of her condition, a multidisciplinary approach to management was deemed necessary.
It is important to emphasize that the overall risk of mortality in PTC is low, and most individuals respond well to appropriate management and treatment. However, studies on patients enrolled in the intracranial hypertension registry show patients with PTC possess a significantly elevated risk of death attributed to suicide and accidental overdose when compared to the general population.[
CONCLUSION
This case underscores the importance of early diagnosis and management of IIH, particularly in patients with PCOS who present with chronic headaches. In fact, in the course of reviewing this manuscript and learning its objective for timely diagnosis of IIH among PCOS patients, one of our health-care providers encountered a 22-year-old patient with a history of PCOS seeking treatment at our neurology clinic due to recent complaints of debilitating headaches and blurry vision. After a comprehensive evaluation, we expeditiously arrived at the diagnosis of IIH.
The patient exhibited clinical signs of papilledema along with an elevated opening pressure of 36 mm H2O during the lumbar puncture procedure. In accordance with our diagnosis, an appropriate therapeutic regimen was promptly initiated, comprising Diamox 250 mg twice daily and Topamax 25 mg. Recognizing the complexity of this case, the patient was referred to both an ophthalmologist and a neurosurgeon for further evaluation and consideration of treatment options. Following thorough discussions and shared decision-making, the patient opted to postpone the consideration of a VP shunting procedure, electing instead to pursue a treatment plan involving medication management and a focused approach to weight loss. Subsequent follow-up visits revealed significant clinical improvement, with resolution of the papilledema and notable improvement of the patient’s previously reported visual disturbances and blurry vision. We find significant reassurance in the patient’s response to treatment, emphasizing the paramount importance of timely diagnosis and intervention in cases of IIH, particularly among patients with PCOS and a history of headaches. Prompt recognition and treatment are crucial to prevent potential complications and optimize patient outcomes.
Furthermore, this case report aims to investigate the pathophysiological mechanisms underlying PCOS, sensorineural hearing loss, ES, and elevated inflammatory markers in a patient diagnosed with IIH. Obesity-induced chronic inflammation and dysregulation of cortisol metabolism may contribute to the pathogenesis of IIH in these individuals.
A multidisciplinary approach involving neurologists, ophthalmologists, endocrinologists, and rheumatologists is essential for the comprehensive management of IIH and its associated comorbidities. Further research and studies are needed to enhance our understanding of the underlying mechanisms and to identify more effective treatment strategies for this challenging condition.
It is crucial to recognize the potential psychological impact of IIH on patients, as studies have shown an increased risk of mortality attributed to suicide and accidental overdose. Addressing the mental health needs of patients with IIH is an integral part of their overall care. Overall, this case report contributes to the existing literature on the clinical presentation, diagnosis, and management of IIH, emphasizing the importance of a multidisciplinary approach and individualized treatment strategies for optimal patient outcomes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Best J, Silvestri G, Burton B, Foot B, Acheson J. The incidence of blindness due to idiopathic intracranial hypertension in the UK. Open Ophthalmol J. 2013. 7: 26-9
2. Bouffard MA. Fulminant idiopathic intracranial hypertension. Curr Neurol Neurosci Rep. 2020. 20: 8
3. Brodsky MC, Vaphiades M. Magnetic resonance imaging in pseudotumor cerebri. Ophthalmology. 1998. 105: 1686-93
4. Çoban K, Aydın E, Özlüoğlu LN. Audio-vestibular findings in increased intracranial hypertension syndrome. J Int Adv Otol. 2017. 13: 100-4
5. Cosar E, Cosar M, Köken G, Sahin FK, Caliskan G, Haktanir A. Polycystic ovary syndrome is related to idiopathic intracranial hypertension according to magnetic resonance imaging and magnetic resonance venography. Fertil Steril. 2008. 89: 1245-6
6. Dave SB, Subramanian PS. Pseudotumor cerebri: An update on treatment options. Indian J Ophthalmol. 2014. 62: 996-8
7. Durcan FJ, Corbett JJ, Wall M. The incidence of pseudotumor cerebri. Population studies in Iowa and Louisiana. Arch Neurol. 1988. 45: 875-7
8. Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013. 81: 1159-65
9. Giuseffi V, Wall M, Siegel PZ, Rojas PB. Symptoms and disease associations in idiopathic intracranial hypertension (Pseudotumor cerebri): A case-control study. Neurology. 1991. 41: 239-44
10. Hermes SM, Miller NR, Waslo CS, Benes SC, Tanne E. Mortality among patients with idiopathic intracranial hypertension enrolled in the IH registry. Neurology. 2020. 95: e921-9
11. Kyung SE, Botelho JV, Horton JC. Enlargement of the sella Turcica in pseudotumor cerebri. J Neurosurg. 2014. 120: 538-42
12. McCluskey G, Doherty-Allan R, McCarron P, Loftus AM, McCarron LV, Mulholland D. Meta-analysis and systematic review of population-based epidemiological studies in idiopathic intracranial hypertension. Eur J Neurol. 2018. 25: 1218-27
13. Mollan SP, Davies B, Silver NC, Shaw S, Mallucci CL, Wakerley BR. Idiopathic intracranial hypertension: Consensus guidelines on management. J Neurol Neurosurg Psychiatry. 2018. 89: 1088-100
14. Mondragon J, Klovenski V, editors. Pseudotumor cerebri. StatPearls. Treasure Island, FL: StatPearls Publishing; 2022. p.
15. Rudnick E, Sismanis A. Pulsatile tinnitus and spontaneous cerebrospinal fluid rhinorrhea: Indicators of benign intracranial hypertension syndrome. Otol Neurotol. 2005. 26: 166-8
16. Satti SR, Leishangthem L, Chaudry MI. Meta-analysis of CSF diversion procedures and dural venous sinus stenting in the setting of medically refractory idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2015. 36: 1899-904
17. Sinclair AJ, Burdon MA, Nightingale PG, Ball AK, Good P, Matthews TD. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: Prospective cohort study. BMJ. 2010. 341: c2701
18. Sørensen PS, Krogsaa B, Gjerris F. Clinical course and prognosis of pseudotumor cerebri. A prospective study of 24 patients. Acta Neurol Scand. 1988. 77: 164-72
19. Spitze A, Lam P, Al-Zubidi N, Yalamanchili S, Lee AG. Controversies: Optic nerve sheath fenestration versus shunt placement for the treatment of idiopathic intracranial hypertension. Indian J Ophthalmol. 2014. 62: 1015-21
20. Sudhakar P. Commentary: The role of inflammation in idiopathic intracranial hypertension. Indian J Ophthalmol. 2021. 69: 1506-7