- Department of Neurosurgery, Città della Salute e della Scienza di Torino, Italy.
- Department of Pathology, University of Turin, Turin, Italy.
Correspondence Address:
Giuseppe Di Perna
Department of Neurosurgery, Città della Salute e della Scienza di Torino, Italy.
DOI:10.25259/SNI_650_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Giuseppe Di Perna1, Fabio Cofano1, Roberto Altieri1, Bianca Maria Baldassarre1, Luca Bertero2, Francesco Zenga1, Diego Garbossa1. III cranial nerve cavernous malformation: A case report and review of the literature. 22-Dec-2020;11:452
How to cite this URL: Giuseppe Di Perna1, Fabio Cofano1, Roberto Altieri1, Bianca Maria Baldassarre1, Luca Bertero2, Francesco Zenga1, Diego Garbossa1. III cranial nerve cavernous malformation: A case report and review of the literature. 22-Dec-2020;11:452. Available from: https://surgicalneurologyint.com/surgicalint-articles/10476/
Abstract
Background: Cavernous malformations generally occur in brain parenchyma but rarely these lesions arise from cranial nerves (CNs).
Case Description: This paper described a case of a woman presented with III CN dysfunction due to the presence of a right III CN cavernoma. Surgical treatment with nerve sparing gross total resection was performed. A 3-month follow-up was documented.
Conclusion: Only few cases of CNs cavernomas have been described in the literature. These lesions have been described to show a more aggressive behavior compared to intraparenchymal cavernomas, especially in symptomatic patients. Differential diagnosis and surgical treatment could be challenging, especially trying to preserve nerve integrity and function.
Keywords: Cavernoma, Cranial nerve, Gross total resection, Nerve sparing, Third nerve palsy
INTRODUCTION
Cavernous malformations (CMs) are vascular lesions formed by abnormally large collections of vascular channels which are typically characterized by a low flow – then hidden at angiography – and absence of interposed neural tissue.[
Rarely, these lesions can arise from cranial nerves (CNs) and their incidental detection raises problems due to differential diagnosis (DD) and treatment, since there is a lack of data in literature, especially about asymptomatic patients.[
CASE REPORT
A 67-year-old woman was referred to our institution for incomplete right III CN palsy. Gadolinium magnetic resonance imaging (MRI) showed an enhanced lesion on the right posterior clinoid process region with extension to the interpeduncular and crural cisterns and mass effect on the III CN. T1- and T2-weighted images were characterized by a mixed intensity while the enhancement after gadolinium administration was homogeneous [
Considering symptoms, characteristics, and location, and after thorough discussion with the patient, a surgical exploration was performed. Surgical strategy was characterized by a right pterional approach. Extradural clinoidectomy – using the high-speed drill – was performed, considering the unknown nature of the lesion. Therefore, a wide proximal control of the right internal carotid artery and also a good control of the cavernous sinus were achieved. After dural opening, Sylvian fissure was split in a distal to proximal fashion. After having reached optic-carotid cistern and followed the posterior communicating artery (PCoA), a brown and blackberry-like lesion was found, strictly adherent to the III CN [
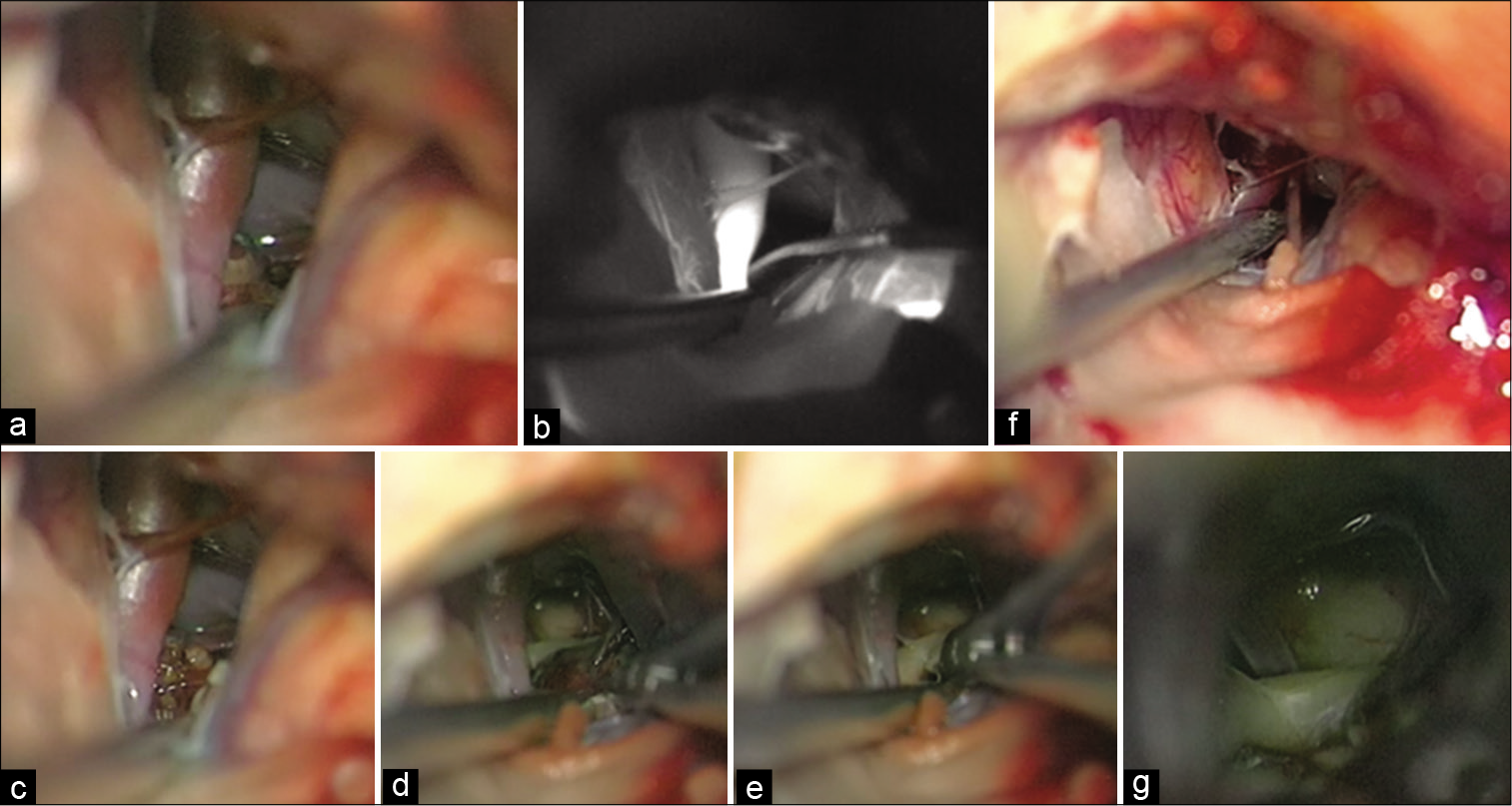
Figure 2:
Intraoperative images (a-g). (a) A right pterional approach was performed and optic-carotid cistern was opened to identify the right internal carotid artery (ICA). Then, (b) intraoperative green video angiography confirmed that the lesion did not arise from the Willis circle. (c) A blackberry-like lesion was observed behind the medial wall of the right ICA, and, after a sharp dissection of the surrounding arachnoid membranes, (d) the strict relationship of the lesion with the right III cranial nerve was observed. (e) Thus, dissection from the nerve was performed and then the lesion was removed. (f and g) At the end of the procedure, the III cranial nerve was spared.
Then, a careful removal of the lesion with anatomical preservation of the III CN was performed [
DISCUSSION
CMs are considered to be vascular malformations with a prevalence of 0.4–0.9% within the general population and a trend of growth because of their recurrent internal hemorrhages.[
There are only few cases of CN CMs described in the recent literature.[
One of the most interesting aspects of this lesion is the fact that radiological images are not always pathognomonic. It makes challenging their DD before surgery, especially when there are no signs of bleeding, since the most frequent lesions along CNs course are schwannomas.[
Since subtotal resection (STR) can lead to the recurrence of the malformation, gross total resection (GTR) is strongly recommended when possible.[
The possibility of removing CNs CMs sparing the nerve is debatable and depends on different factors such as clinical presentation, previous bleeding, degree of adhesion to the nerves, and probably the origin of the lesion.[
Rotondo et al. described 90 cases of CNs CMs from 1979 to 2013. A total number of eight cases of III CN CMs were reported [
In 2007, Itshayek et al. described a case of a young woman presenting with facial pain controlled by pharmacological therapy. The MRI showed the presence of a CMs in the clinoid region. During the procedure, senior surgeon decided not to proceed with the resection since no relationships with the branches of the V nerve were found, while the proximity to the III nerve was considered risky for new postoperative deficits.[
In our case report, the lesion was easily detachable so a GTR with nerve sparing was achieved and also a clinical stability was registered at follow-up.
Another aspect could be the choice of surgical approach. In this case, an extradural anterior clinoidectomy was performed to gain good control to the cavernous sinus and proximal control of the carotid artery in case of intraoperative complications. Another option that could have been taken into account would have been a trans-Sylvian approach to the interpeduncular cistern. The possibility to obtain a wider access to the basal cisterns and to the median aspect of temporal lobe has been described in different studies. Nevertheless, also the risk of middle cerebral artery injury or vasospasm has been reported.[
To summarize, all symptomatic lesions should be treated.[
The role of stereotactic radiosurgery, both as an alternative and as an additional option, appears to be problematic in the management of CN CMs.[
A further consideration consists in the use of intraoperative neuromonitoring (IONM) to guarantee better control of CN function during surgery. Hariharan et al.[
CONCLUSION
CN CMs are rare lesions, and DD could be challenging. Microsurgical excision could be considered as safe and effective to prevent new deficits or in symptomatic patients with high risk of rebleeding. STR could lead to recurrences, a GTR with preservation of nerve integrity should be the target of the treatment.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Campero A, Ajler P, Garategui L, Goldschmidt E, Martins C, Rhoton A. Pterional transsylvian-transinsular approach in three cavernomas of the left anterior mesiotemporal region. Clin Neurol Neurosurg. 2015. 130: 14-9
2. Cofano F, Marengo N, Pecoraro F, Penner F, Bertero L, Zenga F. Spinal epidural capillary hemangioma: Case report and review of the literature. Br J Neurosurg. 2019. p. 1-4
3. de Champfleur NM, Langlois C, Ankenbrandt WJ, le Bars E, Leroy MA, Duffau H. Magnetic resonance imaging evaluation of cerebral cavernous malformations with susceptibility-weighted imaging. Neurosurgery. 2011. 68: 641-7
4. Deshmukh VR, Albuquerque FC, Zabramski JM, Spetzler RF, Steinberg GK, Bricolo A. Surgical management of cavernous malformations involving the cranial nerves. Neurosurgery. 2003. 53: 352-7
5. Hariharan P, Balzer JR, Anetakis K, Crammond DJ, Thirumala PD. Electrophysiology of extraocular cranial nerves: Oculomotor, trochlear, and abducens nerve. J Clin Neurophysiol. 2018. 35: 11-5
6. Huang YC, Tseng CK, Chang CN, Wei KC, Liao CC, Hsu PW. LINAC radiosurgery for intracranial cavernous malformation: 10-year experience. Clin Neurol Neurosurg. 2006. 108: 750-6
7. Itshayek E, Perez-Sanchez X, Cohen JE, Umansky F, Spektor S. Cavernous hemangioma of the third cranial nerve: Case report. Neurosurgery. 2007. 61: E653
8. Iwai Y, Yamanaka K, Nakajima H, Miyaura T. Cavernous angioma of the optic chiasm: Case report. Neurol Med Chir (Tokyo). 1999. 39: 617-20
9. Maiodna E, Ahmad FU, Morcos JJ. Cavernous malformation of the seventh cranial nerve: Case report and review of literature. World Neurosurg. 2016. 91: 676.e13-21
10. Matias-Guiu X, Alejo M, Sole T, Ferrer I, Noboa R, Bartumeus F. Cavernous angiomas of the cranial nerves. Report of two cases. J Neurosurg. 1990. 73: 620-2
11. Ozer E, Kalemci O, Yucesoy K, Canda S. Optochiasmatic cavernous angioma: Unexpected diagnosis. Case report. Neurol Med Chir (Tokyo). 2007. 47: 128-31
12. Potts MB, Chang EF, Young WL, Lawton MT. Transsylviantransinsular approaches to the insula and basal ganglia: Operative techniques and results with vascular lesions. Neurosurgery. 2012. 70: 824-34
13. Robinson RJ, Bhuta S. Susceptibility-weighted imaging of the brain: Current utility and potential applications. J Neuroimaging. 2011. 21: e189-204
14. Rotondo M, Natale M, D’Avanzo R, Pascale M, Scuotto A. Cavernous malformations isolated from cranial nerves: Unexpected diagnosis?. Clin Neurol Neurosurg. 2014. 126: 162-8
15. Samii M, Nakamura M, Mirzai S, Vorkapic P, Cervio A. Cavernous angiomas within the internal auditory canal. J Neurosurg. 2006. 105: 581-7
16. Sürücü O, Sure U, Mittelbronn M, Meyermann R, Becker R. Cavernoma of the trochlear nerve. Clin Neurol Neurosurg. 2007. 109: 791-3