- Department of Pediatrics, Texas Tech University Health Sciences Center, Lubbock, Texas, United States
- School of Medicine, Texas Tech University Health Sciences Center, Lubbock, Texas, United States
- Department of Neurosurgery, University of Oklahoma, Oklahoma City, Oklahoma, United States
- Department of Mathematics, University of Texas Permian Basin, Odessa, Texas, United States.
Correspondence Address:
Ryan D. Morgan, School of Medicine, Texas Tech University Health Sciences Center, Lubbock, Texas, United States.
DOI:10.25259/SNI_472_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Laszlo Nagy1, Ryan D. Morgan2, Reagan A. Collins2, Abdurrahman F. Kharbat3, John Garza4, Muhittin Belirgen1. Impact of timing of decompressive craniectomy on outcomes in pediatric traumatic brain injury. 22-Dec-2023;14:436
How to cite this URL: Laszlo Nagy1, Ryan D. Morgan2, Reagan A. Collins2, Abdurrahman F. Kharbat3, John Garza4, Muhittin Belirgen1. Impact of timing of decompressive craniectomy on outcomes in pediatric traumatic brain injury. 22-Dec-2023;14:436. Available from: https://surgicalneurologyint.com/surgicalint-articles/12685/
Abstract
Background: Decompressive craniectomy (DC) can be utilized in the management of severe traumatic brain injury (TBI). It remains unclear if timing of DC affects pediatric patient outcomes. Further, the literature is limited in the risk assessment and prevention of complications that can occur post DC.
Methods: This is a retrospective review over a 10-year period across two medical centers of patients ages 1 month–18 years who underwent DC for TBI. Patients were stratified as acute (24 h) based on timing to DC. Primary outcomes were Glasgow outcome scale (GOS) at discharge and 6-month follow-up as well as complication rates.
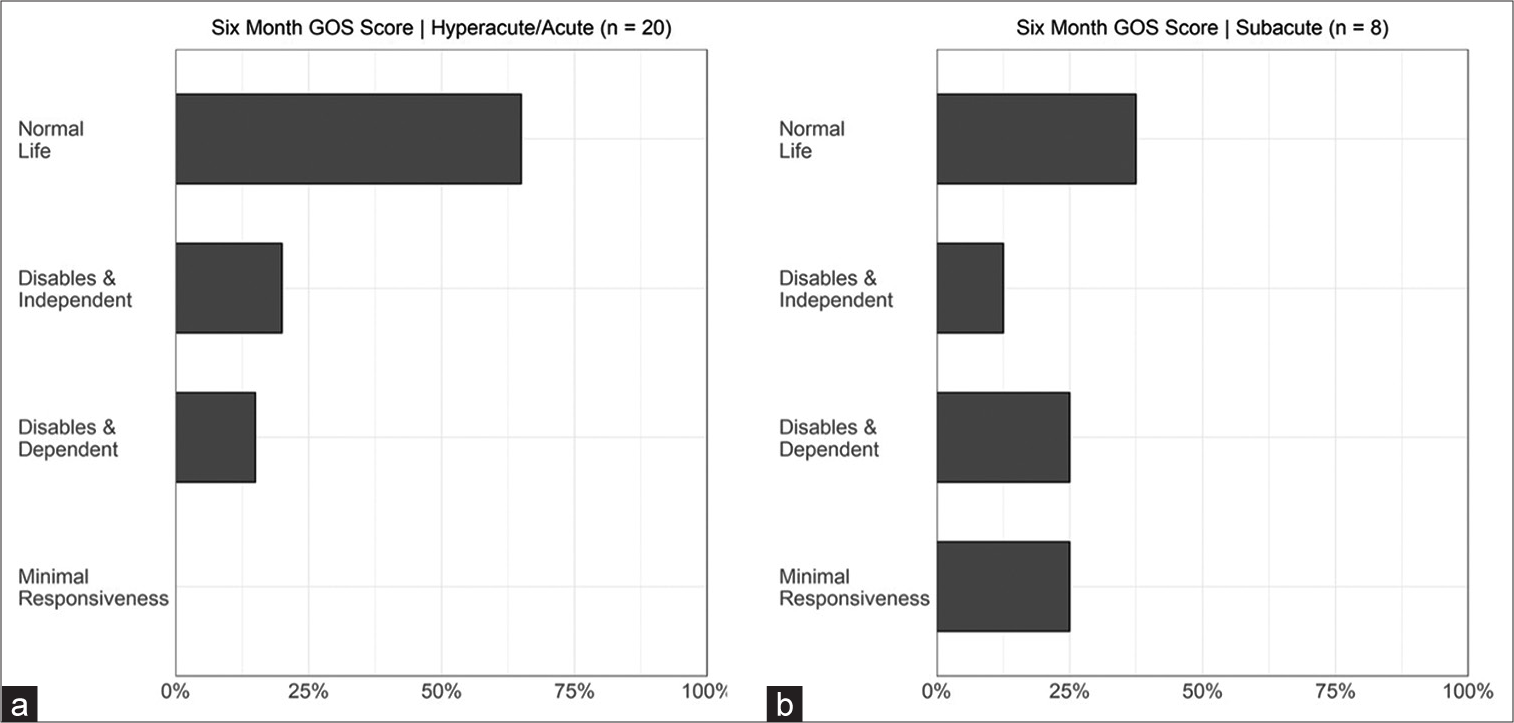
Results: A total of 47 patients fit the inclusion criteria: 26 (55.3%) were male with a mean age of 7.87 ± 5.87 years. Overall, mortality was 31.9% (n = 15). When evaluating timing to DC, 36 (76.6%) patients were acute, and 11 (23.4%) were subacute. Acute DC patients presented with a lower Glasgow coma scale (5.02 ± 2.97) compared to subacute (8.45 ± 4.91) (P = 0.030). Timing of DC was not associated with GOS at discharge (P = 0.938), 3-month follow-up (P = 0.225), 6-month follow-up (P = 0.074), or complication rate (P = 0.505). The rate of posttraumatic hydrocephalus following DC for both groups was 6.4% (n = 3).
Conclusion: Although patients selected for the early DC had more severe injuries at presentation, there was no difference in outcomes. The optimal timing of DC requires a multifactorial approach considered on a case-by-case basis.
Keywords: Cranioplasty, Decompressive craniectomy, Posttraumatic hydrocephalus, Traumatic brain injury
INTRODUCTION
For decades, the leading cause of morbidity and mortality in the pediatric population has been traumatic brain injury (TBI).[
Treatment of severe TBI is divided into first tier and secondary tier therapies. Current first and secondary tier therapies are based on the third edition of guidelines for the management of pediatric severe TBI by Kochanek et al.[
DC is a potentially life saving procedure that is used in the treatment of severe TBI. It is utilized on a case-by-case basis for treatment of severe cerebral edema and intractable ICP.[
DC comes with inherent risks, so it is commonly utilized as a second-tier therapy. However, some recent studies indicate that DC in children can be done effectively and safely.[
MATERIALS AND METHODS
Study population
Following Institutional Review Board approval, we performed a retrospective review of pediatric patients who underwent DC from 2012 to 2021. This study was conducted at Covenant Women and Children’s Hospital and University Medical Center, a Level I Trauma Center that serves as the primary teaching hospital and tertiary referral center for a large rural population. Patients included were younger than 18 years of age and underwent a DC for TBI at one of our institutions. Patients who received a DC for an indication other than TBI were excluded from the study. We obtained a list of patients from the IT department at each institution of patients 18 years or under who presented to the emergency department (ED) for TBI. This list was then manually screened, and all patients who had DC for TBI with or without hematoma evacuation were included in the study.
Data acquisition
All data were extracted from the electronic medical record. Demographic data, including age, sex, mechanism of injury, and type of injury, were obtained for all patients. ICP, CPP, and Glasgow coma scale (GCS) scores were obtained from chart notes at admission, pre-surgery, and post-surgery. Admission data were collected from the first recorded measurement following arrival at our institution. Some patients, especially those in the acute DC group, did not have admission/preoperative ICP or CPP measurements; however, all had admission GCS scores documented. Pre-surgery data were obtained from documentation immediately prior to the operation. Post-surgery data were obtained from notes between 24 and 48 hours after the operation based on when the first postoperative measurements were documented. For pre-surgery ICP measurements in patients who did not receive ICP monitoring before surgery, some patients had ICP monitors placed in the operating room prior to the DC, and these measurements were utilized. Not all patients had ICP and CPP monitoring before surgery; however, all were measured following their operation.
Data were also collected regarding the type of neurologic treatment patients received before surgery and the locations where they received this treatment. Possible treatment locations included the ED, the pediatric intensive care unit (PICU), either outside the hospital or by emergency medical services, and the surgical intensive care unit. The type of DC received, the timing (acute versus subacute) of the DC, and the indications for the operation were documented for each patient. Preoperative imaging data included computed tomography (CT) and magnetic resonance imaging (MRI). CT studies were utilized for scoring the Rotterdam score and Marshal classification. Imaging studies were also utilized for the description of mass lesions before surgery and progression following DC. After the operation, complications, including any incidence of infection, CSF leak, hydrocephalus, subdural/subgaleal fluid collection, and new or progression of hematomas, were recorded from imaging studies and neurosurgical notes for each patient. Hydrocephalus was defined as ventriculomegaly requiring shunting following the DC. Complications were noted, whether a follow-up operation was indicated or not, as some complications can delay operations such as cranioplasty. Significant progression of cerebral swelling and herniation through the skull defect was also documented based on imaging studies and neurosurgical notes. The usage of drains, the type of drains, and the amount of CSF drained from each patient were also documented.
Primary outcomes included total length of stay (LOS), ICU LOS, and Glasgow outcome scale (GOS). GOS was utilized as it has previously been validated in head injury and has been used in other similar studies.[
All DC procedures and the decision to operate were decided on a case-by-case basis with a multifactorial decision-making method. The decision to operate preoperative medical management was guided by the 2019 TBI guidelines by Kochanek et al.[
Statistical analysis
Statistical analyses used two-sided P-values, independent samples, and a significance level of α = 0.05. Continuous variables are summarized using the mean and standard deviation (SD). Categorical variables are summarized using counts and percentages. Differences in independent interval level variables are tested using the permutational unequal variance Welch t-test based on 1000 permutations. Differences in independent nominal level and binary variables are tested using Fisher’s test. The standardized mean difference is used as the standardized effect size for nominal-level, binary, and interval-level variables. Differences between ordinal level variables are tested using the Mann–Whitney U-test with Cliff’s δ as the standardized effect size. Associations between independent interval or ordinal level variables are tested using Spearman correlation. For repeated measures pre-surgery to post-surgery, the permutational dependent samples t-test or the sign test has been applied. To aid the interpretation of nonsignificant hypothesis tests, Supplementary
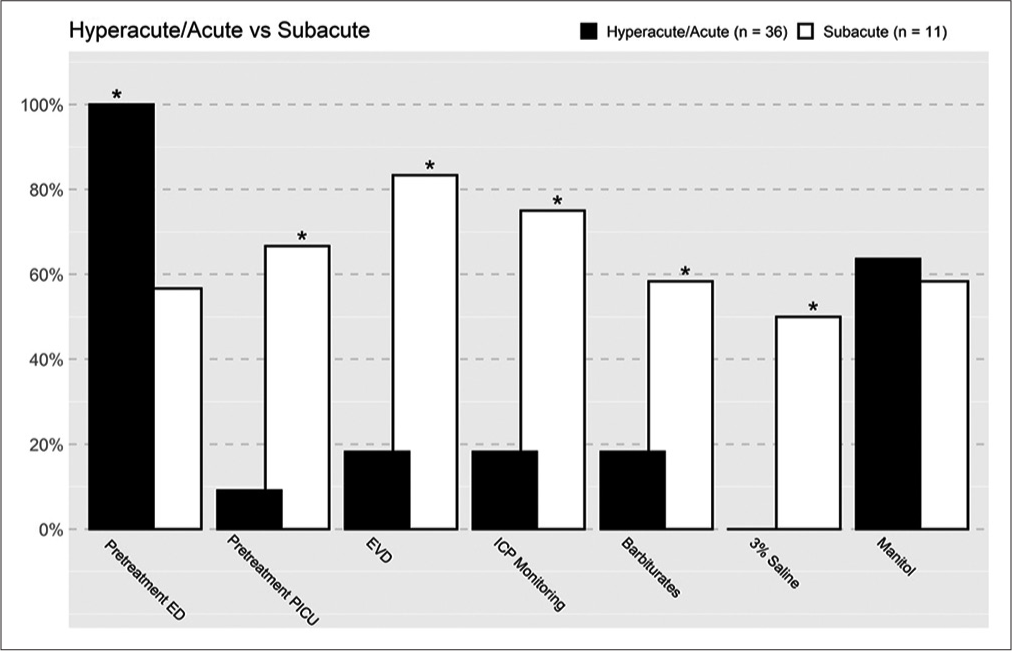
Figure 1:
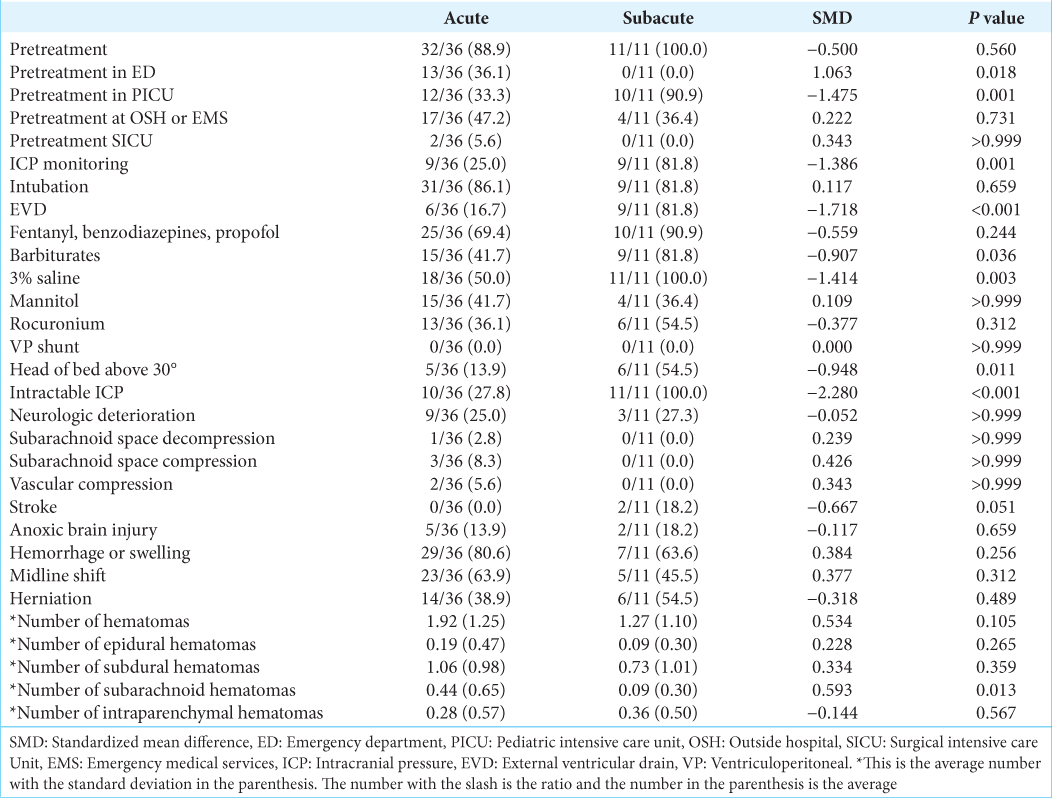
Differences in pretreatment methods/location between the acute and subacute groups. Asterix refers to statistical significance, if there is an asterix statistical significance was met. ED: Emergency department, PICU: Pediatric intensive care unit, EVD: External ventricular drain, ICP: Intracranial pressure.
RESULTS
Demographics
A total of 47 patients underwent a DC; 25 (53.2%) were male, and the mean age was 7.87 (SD = 5.87) years. The most common etiology of these patients was motor vehicle collision (42.6%), followed by known accidental trauma (21.3%), nonaccidental trauma (19.1%), and gunshot wounds (17.0%). The overall mortality rate was 31.9% (n = 15). The average admission GCS for all patients was 5.8 (SD = 3.7). The mean GOS of the survivors was 3.68 (SD = 1.06) at discharge and 4.25 (1.00) at six-month follow-up, with 75.0% of the survivors having a favorable outcome (GOS 4 or 5) at their 6-month follow-up.
Timing of DC
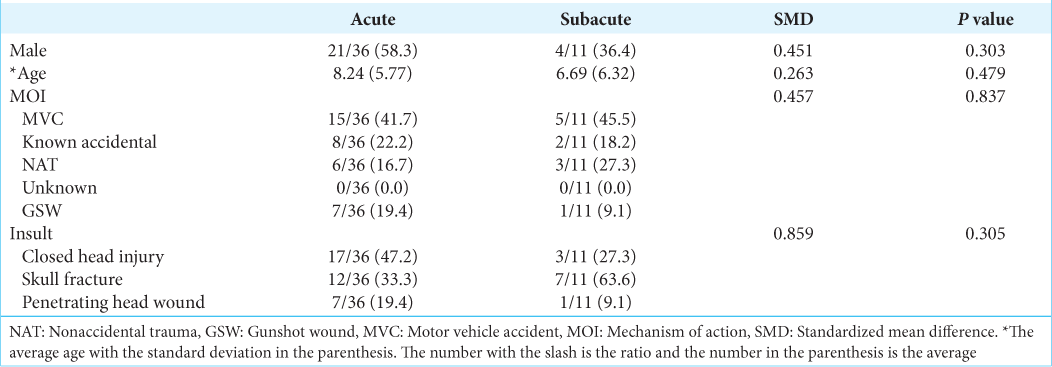
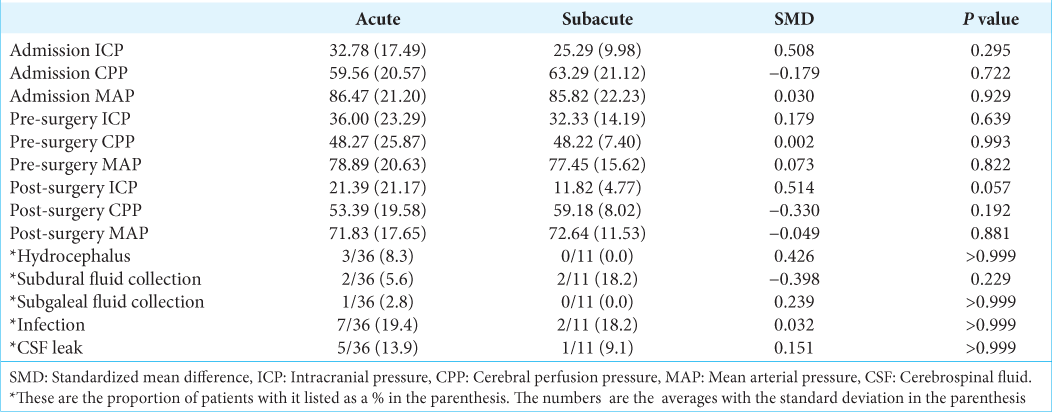
There were 36 (76.6%) patients who underwent an acute procedure, whereas 11 (23.4%) underwent a subacute procedure. There were no statistically significant differences in age, sex, mechanism of injury, or original insult between the two groups [
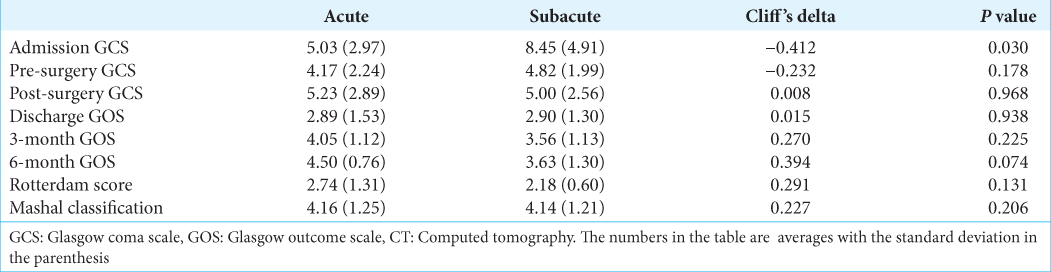
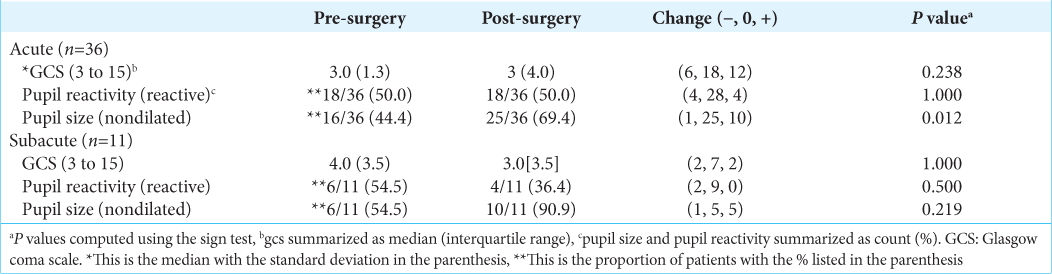
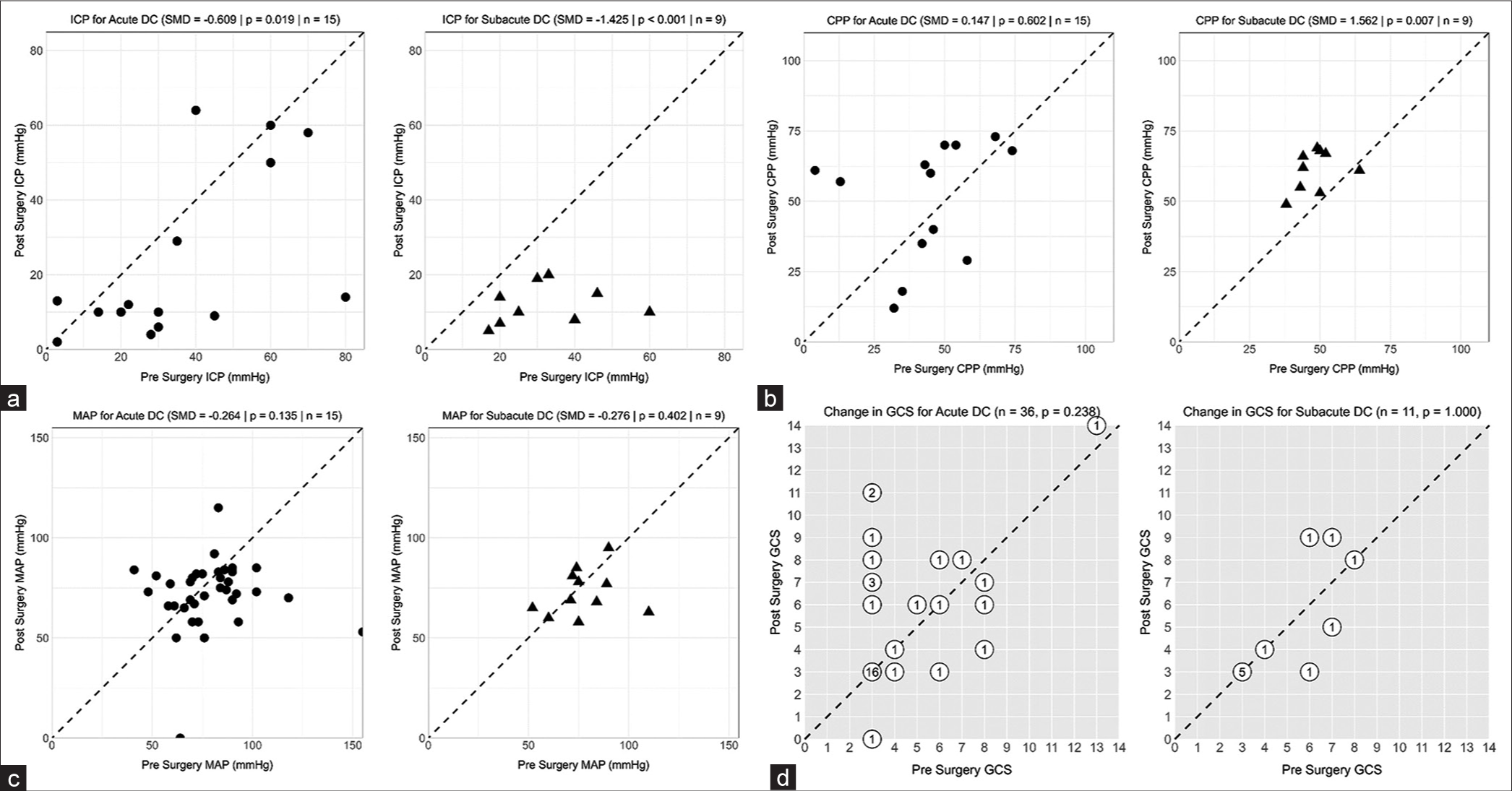
The acute group presented with lower GCS scores than the subacute group (P = 0.030) [
Outcomes
The overall complication rate was 36.6% [
Figure 3:
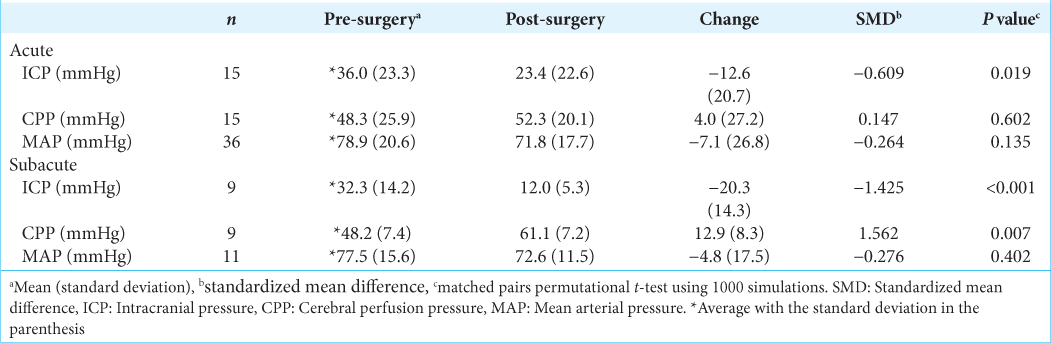
Pre- and post-surgery characteristics in the acute versus subacute decompressive craniectomy groups. (a) Pre- and post-surgery intracranial pressure. (b) Pre- and post-surgery cerebral perfusion pressure. (c) Pre- and post-surgery mean arterial pressure. (d) Pre- and post-surgery Glasgow coma scale. ICP: Intracranial pressure, DC: Decompressive craniectomy, SMD: Standardized mean difference, GCS: Glasgow coma scale, MAP: Mean arterial pressure, CPP: Cerebral perfusion pressure. The numbers correlate to the number of patients who shared the same point.
Drain usage
Of all the patients who underwent DC, 26 (55.3%) had an EVD, and 39 (83.0%) had a subgaleal drain postoperatively. The average time for a patient to have the subgaleal drain in place was 3.12 (SD = 2.34) days. Patients who had an EVD had an average of 1.8 EVDs (SD = 0.93) during their stay. The usage of EVDs was associated with an increased rate of infection (P = 0.007), while the number of EVDs used was associated with an increased rate of subdural fluid collections (P = 0.036). Similarly, the number of days spent with a subgaleal drain was associated with an increased incidence of infection (P = 0.040). There was a greater incidence of CSF leak with increasing days with a subgaleal drain, though not reaching statistical significance (P = 0.063). There were no significant differences in subgaleal fluid collection, subdural fluid collection, or hydrocephalus with either EVD or subgaleal drain. The total overall incidence of subgaleal fluid collections was 2.1%, subdural fluid collections were 8.5%, and hydrocephalus was 6.4%. The median amount of CSF drained was 200– 300 mL/24 h. Pressure settings of EVDs fluctuated in patients based on the need for CSF drainage.
DISCUSSION
The role of DC following severe TBI remains heavily debated in the neurosurgery community.[
The impact of timing to DC following the primary insult and hospital arrival remains unclear in the pediatric population.[
Patel et al. found that after controlling for mechanism of injury and injury severity, rural pediatric TBI patients had the same mortality and in hospital complications as compared to urban patients.[
Following the results and limitations of the DECRA and rescue ICP studies, the potential benefits and limitations of DC remain unclear. However, within the pediatric population, many studies report surviving patients with favorable outcomes following DC.[
Controversy surrounding the usage of DC is in part due to the relatively high complication rate.[
Furthermore, in a study by Adamo et al., three patients (50%) developed CSF fistulas after the operation. Following fistula formation, EVDs were inserted to drain excess CSF.[
Despite the high prevalence of PTH, few studies exist on the topic. PTH rates are usually reported to be around 21–40%, with one smaller study by Adamo et al. reporting rates as high as 100%.[
In a study by Carballo-Cuello et al. specifically looking at PTH following DC, they found that PTH was more prominent in patients receiving later cranioplasties when compared to patients undergoing earlier cranioplasties.[
Another commonly encountered complication after DC is subdural and subgaleal hygromas. It is believed that the development of hygromas is linked to the development of PTH. A study by Pechmann et al. supports the notion that all patients who developed PTH had a preexisting subdural hygroma.[
With respect to the use of postoperative EVDs and subgaleal drains, we believe that these are pivotal in reducing complications and aiding in the healing process. Care must be taken in the usage of drains as it has been noted that over-siphoning of CSF can result in slit ventricle syndrome with spikes in ICP secondary to venous engorgement.[
Limitations
A limitation of this study is the retrospective nature, which can include bias due to the lack of randomization. Due to the small sample size coupled with the numerous covariables present in the study, we were not able to perform any multivariable regression analysis of independent predictors of outcome following DC. The sample size of 47 does hold some limitations on the generalizability of the study sample. Type II errors cannot be completely ruled out due to the pilot scale of the study sample. We were unable to capture the exact timing from injury to DC and the exact timing of ICP, CPP, and MAP measurements, which were collected as admission, preoperation, and postoperation. Finally, decision-making was surgeon preference, with four different surgeons operating on the patient population. Due to the pilot size of the study sample, we were able to collect substantial data on each patient. This gave us the benefit of having ample descriptive characteristics that were included within the study. A prospective study is now needed to adequately randomize patients and collect data that are not readily available within patient charts and should be considered for future work.
CONCLUSION
Timing to DC following arrival to the hospital after a TBI did not significantly correlate with better outcomes in the pediatric population. Pediatric patients have further demonstrated positive outcomes following DC, and our rates of PTH are the lowest recorded. This further demonstrates the safety and efficacy of DC in children following severe traumatic brain injuries; however, more studies with increased patient populations are needed.
Ethical approval
The author(s) declare that they have taken the ethical approval from IRB. (IRB approval number #00000096, 7/24/2020).
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Adamo MA, Drazin D, Smith C, Waldman JB. Comparison of accidental and nonaccidental traumatic brain injuries in infants and toddlers: Demographics, neurosurgical interventions, and outcomes. J Neurosurg Pediatr. 2009. 4: 414-9
2. Adamo MA, Drazin D, Waldman JB. Decompressive craniectomy and postoperative complication management in infants and toddlers with severe traumatic brain injuries. J Neurosurg Pediatr. 2009. 3: 334-9
3. Ardissino M, Tang A, Muttoni E, Tsang K. Decompressive craniectomy in paediatric traumatic brain injury: A systematic review of current evidence. Childs Nerv Syst. 2018. 35: 209-16
4. Ballestero MF, Furlanetti LL, Augusto LP, Chaves PH, Santos MV, de Oliveira RS. Decompressive craniectomy for severe traumatic brain injury in children: Analysis of long-term neuropsychological impairment and review of the literature. Childs Nerv Syst. 2019. 35: 1507-15
5. Bruns N, Kamp O, Lange K, Lefering R, Felderhoff-Müser U, Dudda M. Functional short-term outcomes and mortality in children with severe traumatic brain injury: Comparing decompressive craniectomy and medical management. J Neurotrauma. 2022. 39: 944-53
6. Carballo-Cuello C, de Jesus O, Fernandez-de Thomas RJ, Garcia M, Vigo-Prieto J, de Jesus-Espinosa A. Posttraumatic hydrocephalus in pediatric patients after decompressive craniectomy. World Neurosurg. 2020. 136: e690-4
7. Csókay A, Emelifeonwu JA, Fügedi L, Valálik I, Láng J. The importance of very early decompressive craniectomy as a prevention to avoid the sudden increase of intracranial pressure in children with severe traumatic brain swelling (retrospective case series). Childs Nerv Syst. 2011. 28: 441-4
8. Csókay A, Nagy L, Pentelénvi T. “Vascular tunnel” formation to improve the effect of decompressive craniectomy in the treatment of brain swelling caused by trauma and hypoxia. Acta Neurochir (Wien). 2001. 143: 173-5
9. Cushing H. I. Subtemporal decompressive operations for the intracranial complications associated with bursting fractures of the skull. Ann Surg. 1908. 47: 641-4.1
10. Elsawaf Y, Anetsberger S, Luzzi S, Elbabaa SK. Early decompressive craniectomy as management for severe traumatic brain injury in the pediatric population: A comprehensive literature review. World Neurosurg. 2020. 138: 9-18
11. Figaji AA, Fieggen AG, Argent AC, Le Roux PD, Peter JC. Intracranial pressure and cerebral oxygenation changes after decompressive craniectomy in children with severe traumatic brain injury. Acta Neurochir Suppl. 2008. 102: 77-80
12. Güresir E, Schuss P, Seifert V, Vatter H. Decompressive craniectomy in children: Single-center series and systematic review. Neurosurgery. 2012. 70: 881-8
13. Jagannathan J, Okonkwo DO, Dumont AS, Ahmed H, Bahari A, Prevedello DM. Outcome following decompressive craniectomy in children with severe traumatic brain injury: A 10-year single-center experience with long-term follow up. J Neurosurg. 2007. 106: 268-75
14. Josan VA, Sgouros S. Early decompressive craniectomy may be effective in the treatment of refractory intracranial hypertension after traumatic brain injury. Childs Nerv Syst. 2006. 22: 1268-74
15. Kan P, Amini A, Hansen K, White GL, Brockmeyer DL, Walker ML. Outcomes after decompressive craniectomy for severe traumatic brain injury in children. J Neurosurg. 2006. 105: 337-42
16. Khan SA, Shallwani H, Shamim MS, Murtaza G, Enam SA, Qureshi RO. Predictors of poor outcome of decompressive craniectomy in pediatric patients with severe traumatic brain injury: A retrospective single center study from Pakistan. Childs Nerv Syst. 2014. 30: 277-81
17. Kochanek PM, Tasker RC, Bell MJ, Adelson PD, Carney N, Vavilala MS. Management of pediatric severe traumatic brain injury: 2019 consensus and guidelines-based algorithm for first and second tier therapies. Pediatr Crit Care Med. 2019. 20: 269-79
18. Kochanek PM, Tasker RC, Carney N, Totten AM, Adelson PD, Selden NR. Guidelines for the management of pediatric severe traumatic brain injury, third edition: Update of the brain trauma foundation guidelines. Pediatr Crit Care Med. 2019. 20: S1-82
19. Kurland DB, Khaladj-Ghom A, Stokum JA, Carusillo B, Karimy JK, Gerzanich V. Complications associated with decompressive craniectomy: A systematic review. Neurocrit Care. 2015. 23: 292-304
20. Manfiotto M, Beccaria K, Rolland A, Paternoster G, Plas B, Boetto S. Decompressive craniectomy in children with severe traumatic brain injury: A multicenter retrospective study and literature review. World Neurosurg. 2019. 129: e56-62
21. McCowan CL, Swanson ER, Thomas F, Handrahan DL. Outcomes of pediatric trauma patients transported from rural and urban scenes. Air Med J. 2008. 27: 78-83
22. McMillan T, Wilson L, Ponsford J, Levin H, Teasdale G, Bond M. The Glasgow outcome scale-40 years of application and refinement. Nat Rev Neurol. 2016. 12: 477-85
23. Mhanna MJ, Mallah WE, Verrees M, Shah R, Super DM. Outcome of children with severe traumatic brain injury who are treated with decompressive craniectomy. J Neurosurg Pediatr. 2015. 16: 508-14
24. Patel N, West M, Wurster J, Tillman C. Pediatric traumatic brain injuries treated with decompressive craniectomy. Surg Neurol Int. 2013. 4: 128
25. Patel PD, Kelly KA, Chen H, Greeno A, Shannon CN, Naftel RP. Measuring the effects of institutional pediatric traumatic brain injury volume on outcomes for rural-dwelling children. J Neurosurg Pediatr. 2021. 28: 638-46
26. Pechmann A, Anastasopoulos C, Korinthenberg R, van Velthoven-Wurster V, Kirschner J. Decompressive craniectomy after severe traumatic brain injury in children: Complications and outcome. Neuropediatrics. 2015. 46: 5-12
27. Polin RS, Shaffrey ME, Bogaev CA, Tisdale N, Germanson T, Bocchicchio B. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997. 41: 84-92
28. Prasad GL, Gupta DK, Mahapatra AK, Borkar SA, Sharma BS. Surgical results of growing skull fractures in children: A single centre study of 43 cases. Childs Nerv Syst. 2015. 31: 269-77
29. Prasad GL, Gupta DK, Mahapatra AK, Sharma BS. Surgical results of decompressive craniectomy in very young children: A level one trauma centre experience from India. Brain Inj. 2015. 29: 1717-24
30. Rallis D, Poulos P, Kazantzi M, Kalampalikis P. Rescue decompressive craniectomy in children with severe traumatic brain injury. J Pediatr Intensive Care. 2018. 7: 33-8
31. Ruf B, Heckmann M, Schroth I, Hügens-Penzel M, Reiss I, Borkhardt A. Early decompressive craniectomy and duraplasty for refractory intracranial hypertension in children: Results of a pilot study. Crit Care. 2003. 7: R133-8
32. Rutigliano D, Egnor MR, Priebe CJ, McCormack JE, Strong N, Scriven RJ. Decompressive craniectomy in pediatric patients with traumatic brain injury with intractable elevated intracranial pressure. J Pediatr Surg. 2006. 41: 83-7
33. Scollato A, Gallina P, Bahl G, Di Lorenzo N. Decompressive craniectomy arrests pulsatile aqueductal CSF flux: An in vivo demonstration using phase-contrast MRI. Case report. Br J Neurosurg. 2015. 29: 440-2
34. Sood S, Kumar CR, Jamous M, Schuhmann MU, Ham SD, Canady AI. Pathophysiological changes in cerebrovascular distensibility in patients undergoing chronic shunt therapy. J Neurosurg. 2004. 100: 447-53
35. Taylor A, Butt W, Rosenfeld J, Shann F, Ditchfield M, Lewis E. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst. 2001. 17: 154-62
36. Thomale UW, Graetz D, Vajkoczy P, Sarrafzadeh AS. Severe traumatic brain injury in children-a single center experience regarding therapy and long-term outcome. Childs Nerv Syst. 2010. 26: 1563-73
37. Wilson MH. Monro-Kellie 2.0. The dynamic vascular and venous pathophysiological components of intracranial pressure. J Cereb Blood Flow Metab. 2016. 36: 1338-50
38. Zhang D, Xue Q, Chen J, Dong Y, Hou L, Jiang Y. Decompressive craniectomy in the management of intracranial hypertension after traumatic brain injury: A systematic review and meta-analysis. Sci Rep. 2017. 7: 8800