- Department of Neurosurgery, Japan Community Health care Organization Kyushu Hospital, Kitakyushu, Japan.
- Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
- Director General, National Cerebral and Cardiovascular Center Hospital, Suita, Osaka, Japan.
Correspondence Address:
Nobutaka Mukae, Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
DOI:10.25259/SNI_1077_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kotaro Ono1, Nobutaka Mukae2, Ataru Nishimura2, Koichi Arimura2, Masahiro Mizoguchi2, Koji Yoshimoto2, Koji Iihara3. Impaired visual acuity as an only symptom of shunt malfunction, long time after initial cyst-peritoneal shunting for arachnoid cyst: A case report. 25-Feb-2022;13:68
How to cite this URL: Kotaro Ono1, Nobutaka Mukae2, Ataru Nishimura2, Koichi Arimura2, Masahiro Mizoguchi2, Koji Yoshimoto2, Koji Iihara3. Impaired visual acuity as an only symptom of shunt malfunction, long time after initial cyst-peritoneal shunting for arachnoid cyst: A case report. 25-Feb-2022;13:68. Available from: https://surgicalneurologyint.com/surgicalint-articles/11413/
Abstract
Background: Long-term outcomes after surgical treatment of arachnoid cysts (ACs) have not been reported adequately. Impaired visual acuity is not a common symptom of shunt dependency syndrome due to cyst-peritoneal (CP) shunt malfunction for ACs. We report a case of CP shunt malfunction, who presented only impaired visual acuity as a symptom, long after the initial surgical treatment.
Case Description: A 16-year-old boy was surgically treated for the left frontal AC with CP shunting at 2 years of age. Extension of the peritoneal shunt catheter was performed at 15 years of age. A year later, he started experiencing impairment of visual acuity without headaches, which worsened to bilateral light perception. The presence of bilateral optic atrophy was confirmed. The AC in the left frontal lobe had enlarged very slightly, with shortening of the intracystic catheter, and the cerebrospinal fluid pressure was elevated to 30 cmH2O. He was treated with lumboperitoneal shunting. The visual acuity showed limited improvement.
Conclusion: The possibility of CP shunt malfunction and shunt dependency syndrome should be considered, even if the patient presented only impaired visual acuity and no significant changes in the size of the ACs are observed.
Keywords: Cyst-peritoneal shunt, Impaired visual acuity, Increased intracranial pressure
INTRODUCTION
Arachnoid cysts (ACs) are common cystic malformations, frequently occurring at various intracranial locations.[
Herein, we report a case of CP shunt malfunction for AC who presented only impaired visual acuity, as a shunt-dependent syndrome.
CASE REPORT
The patient was a 16-year-old male. At the age of 1 year, his parents noticed the enlarging of his head, and following investigations revealed an AC in the left frontal lobe and hydrocephalus. He was treated with CP shunting at 2 years of age [
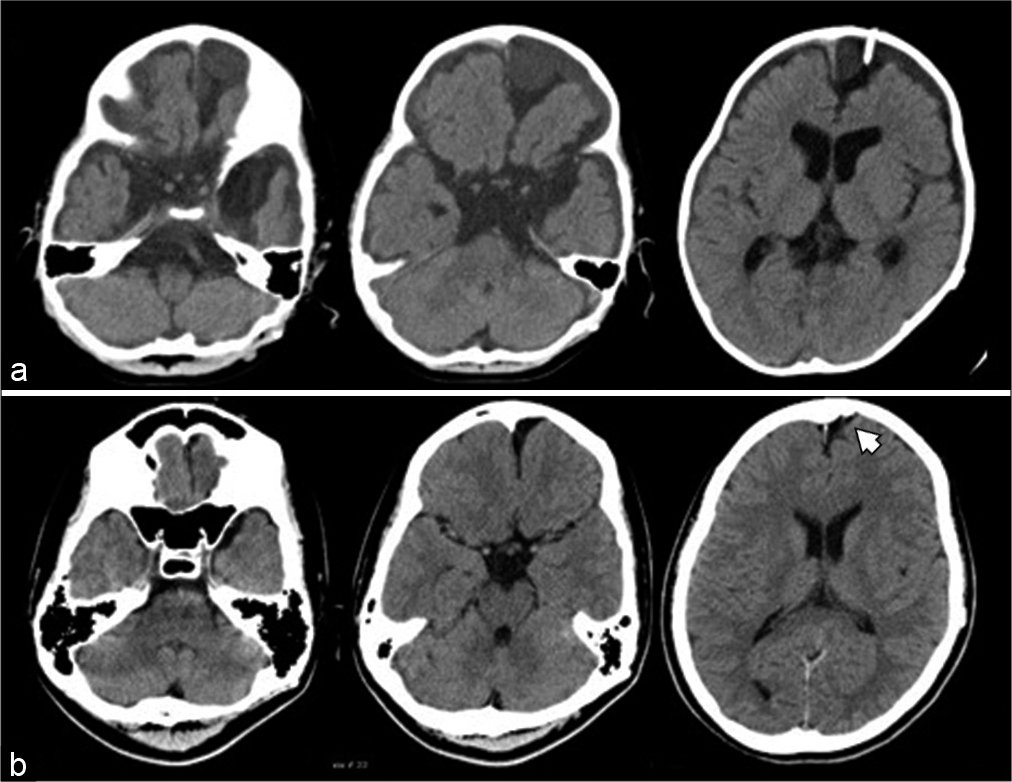
Figure 1:
(a) Postoperative computed tomography (CT) scan after cyst-peritoneal (CP) shunting at 2 years of age showing left frontal arachnoid cyst (AC), dilation of cerebrospinal (CSF) space in bilateral middle cranial fossa, basal cisterns, and lateral ventricles. A CP shunt catheter is inserted in the left frontal AC. (b) CT after surgery for extension of the peritoneal shunt catheter at 15 years of age showing reduced size of the left frontal AC and CSF space in bilateral middle cranial fossa and lateral ventricles, compared to that seen at the age of 2 years. However, the shunt catheter in the left frontal AC is shortened (white arrowhead).
Except impaired visual acuity, he did not show any neurological problems. Fundus examination revealed bilateral optic atrophy [
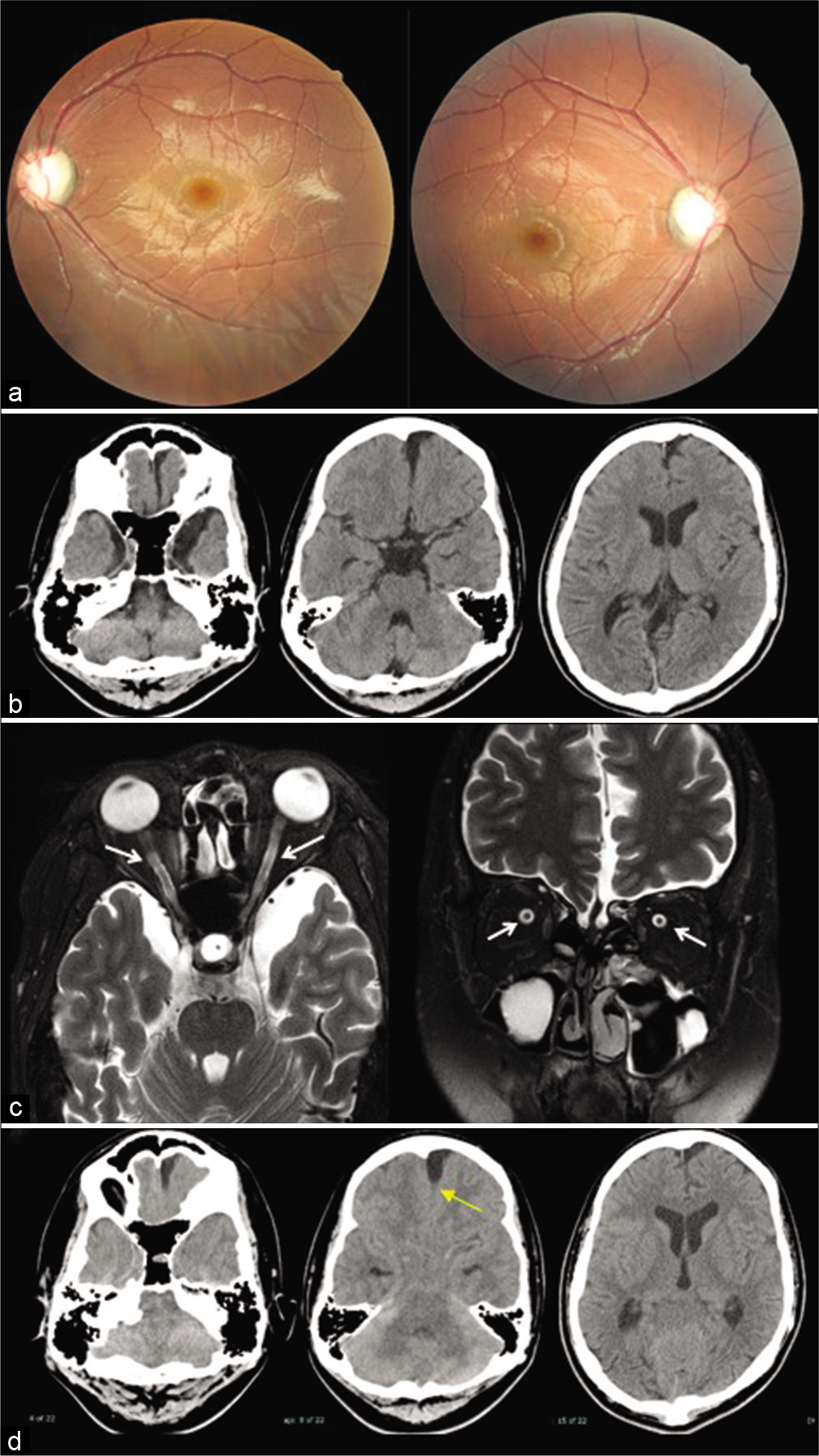
Figure 2:
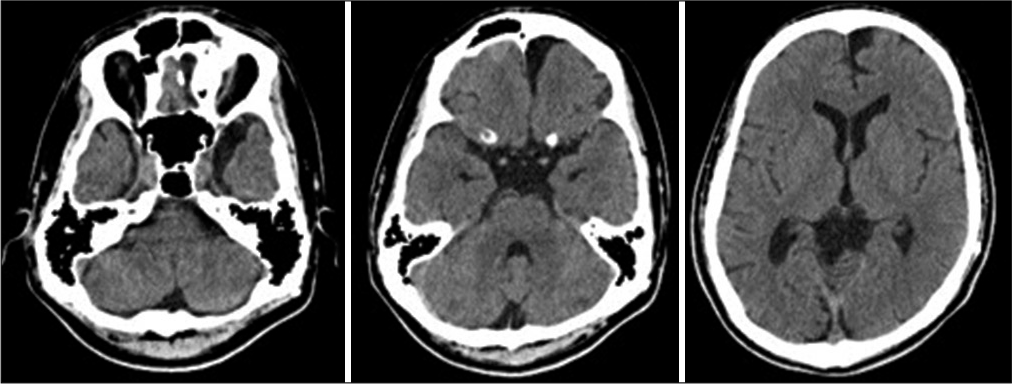
(a) Fundus examination at 16 years of age showing bilateral optic atrophy. (b) Computed tomography after 9 months since the initiation of visual symptoms. Slight enlargement of the left frontal arachnoid cysts (AC) and dilated cerebrospinal fluid (CSF) spaces in bilateral middle cranial fossa and lateral ventricles compared to Figure 1b. (c) Magnetic resonance imaging at 16 years of age demonstrates distention of the CSF space around the optic nerve sheath (white arrows). (d) Cisternography with iotrolan. There is a slight influx of contrast agent in the AC (yellow arrow). Communication between the left frontal AC, CSF spaces in the middle cranial fossa, and basal cisterns is confirmed.
DISCUSSION
The patient we have reported here is thought to have caused elevation of ICP triggered by catheter deviation from AC. We think that this is a very rare case report in that the only symptom was impaired visual acuity. Impaired visual acuity as a symptom of ACs is originally relatively rare, accounting for 1.6–4% of ACs in the literature.[
Increased ICP is a possible mechanism of for the development of impaired visual acuity in this case. In idiopathic intracranial hypertension, impaired visual acuity is quite common.[
Regarding the CT and MRI findings in our case, the changes were modest. Aoki et al. and Sunami et al. reported similar cases of increased ICP as a result of CP shunt malfunction without enlargement of cyst and ventricles, as a slit ventricle syndrome,[
CONCLUSION
Caution should be exercised while treating AC patients with CP shunt developing impaired visual acuity. The possibility of CP shunt malfunction and shunt dependency syndrome should be considered even if the patient presented only impaired visual acuity, and no significant changes in the size of the ACs are observed.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Editage (www.editage.com) for English language editing.
References
1. Al-Holou WN, Yew AY, Boomsaad ZE, Garton HJ, Muraszko KM, Maher CO. Prevalence and natural history of arachnoid cysts in children. J Neurosurg Pediatr. 2010. 5: 578-85
2. Aoki N, Sakai T, Umezawa Y. Slit ventricle syndrome after cyst-peritoneal shunting for the treatment of intracranial arachnoid cyst. Childs Nerv Syst. 1990. 6: 41-3
3. Choi JW, Lee JY, Phi JH, Kim SK, Wang KC. Stricter indications are recommended for fenestration surgery in intracranial arachnoid cysts of children. Childs Nerv Syst. 2015. 31: 77-86
4. Cincu R, Agrawal A, Eiras J. Intracranial arachnoid cysts: Current concepts and treatment alternatives. Clin Neurol Neurosurg. 2007. 109: 837-43
5. Galarza M, Pomata HB, Pueyrredón F, Bartuluchi M, Zuccaro GN, Monges JA. Symptomatic supratentorial arachnoid cysts in children. Pediatr Neurol. 2002. 27: 180-5
6. Gotz-Wieckowska A, Glowka L, Brazert A, Pawlak M. Ophthalmological symptoms in children with intracranial cysts. Sci Rep. 2017. 7: 13630
7. Holbrook J, Saindane AM. Imaging of intracranial pressure disorders. Neurosurgery. 2017. 80: 341-54
8. Huang JH, Mei WZ, Chen Y, Chen JW, Lin ZX. Analysis on clinical characteristics of intracranial Arachnoid Cysts in 488 pediatric cases. Int J Clin Exp Med. 2015. 8: 18343-50
9. Ishii T, Takagi K, Shirouzu M, Watanabe T, Shinohara T, Furuya K. Acute onset of visual disturbance in a patient with arachnoid cyst in the middle cranial fossa. Brain Nerve. 2008. 60: 97-102
10. Jafrani R, Raskin JS, Kaufman A, Lam S. Intracranial arachnoid cysts: Pediatric neurosurgery update. Surg Neurol Int. 2019. 10: 15
11. Kaliaperumal C, O’Connor B, Marks C. Development of intracranial hypertension after surgical management of intracranial arachnoid cyst: report of three cases and review of the literature. World Neurosurg. 2013. 80: 222.e1-4
12. Kim SK, Cho BK, Chung YN, Kim HS, Wang KC. Shunt dependency in shunted arachnoid cyst: A reason to avoid shunting. Pediatr Neurosurg. 2002. 37: 178-85
13. Kural C, Kullmann M, Weichselbaum A, Schuhmann MU. Congenital left temporal large arachnoid cyst causing intraorbital optic nerve damage in the second decade of life. Childs Nerv Syst. 2016. 32: 575-8
14. Li C, Yin L, Jiang T, Ma Z, Jia G. Shunt dependency syndrome after cystoperitoneal shunting of arachnoid cysts. Childs Nerv Syst. 2014. 30: 471-6
15. Osaguona VB. Differential diagnoses of the pale/white/ atrophic disc. Community Eye Health. 2016. 29: 71-4
16. Pradilla G, Jallo G. Arachnoid cysts: Case series and review of the literature. Neurosurg Focus. 2007. 22: E7
17. Rabiei K, Jaraj D, Marlow T, Jensen C, Skoog I, Wikkelsø C. Prevalence and symptoms of intracranial arachnoid cysts: A population-based study. J Neurol. 2016. 263: 689-94
18. Sunami K, Saeki N, Sunada S, Hoshi S, Murai H, Kubota M. Slit ventricle syndrome after cyst-peritoneal shunting for temporal arachnoid cyst in children--a clinical entity difficult to detect on neuroimaging study. Brain Dev. 2002. 24: 776-9
19. Wall M. Update on Idiopathic Intracranial Hypertension. Neurol Clin. 2017. 35: 45-57
20. Wegener M, Prause JU, Thygesen J, Milea D. Arachnoid cyst causing an optic neuropathy in neurofibromatosis 1. Acta Ophthalmol. 2010. 88: 497-9
21. Zhang B, Zhang Y, Ma Z. Long-term results of cystoperitoneal shunt placement for the treatment of arachnoid cysts in children. J Neurosurg Pediatr. 2012. 10: 302-5