- Department of Neurosurgery, International University of Health and Welfare, Narita Hospital,
- Department of Neurosurgery, International University of Health and Welfare, Graduate School of Medicine, Narita, Japan.
Correspondence Address:
Dr. Keisuke Onoda, Department of Neurosurgery, International University of Health and Welfare, Narita Hospital, Narita, Japan.
DOI:10.25259/SNI_494_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Keisuke Onoda1, Ren Fujiwara2, Ryohei Sashida2, Yu Hirokawa2, Tomihiro Wakamiya1, Yuhei Michiwaki1, Tatsuya Tanaka1, Kazuaki Shimoji1, Eiichi Suehiro1, Fumitaka Yamane1, Masatou Kawashima1, Akira Matsuno1. In vivo goat brain model for neurosurgical training. 05-Aug-2022;13:344
How to cite this URL: Keisuke Onoda1, Ren Fujiwara2, Ryohei Sashida2, Yu Hirokawa2, Tomihiro Wakamiya1, Yuhei Michiwaki1, Tatsuya Tanaka1, Kazuaki Shimoji1, Eiichi Suehiro1, Fumitaka Yamane1, Masatou Kawashima1, Akira Matsuno1. In vivo goat brain model for neurosurgical training. 05-Aug-2022;13:344. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11762
Abstract
Background: Novice neurosurgeons require neurosurgical technique training, but the current method is demanding and time consuming. Therefore, it is crucial to perform training using an appropriate and informative method. In this report, we describe our attempts to provide training in neurosurgical techniques using goat in vivo brain model and to demonstrate the effectiveness of this model.
Methods: Under general anesthesia, the surgery was performed on a male goat in the prone position. A midline liner skin incision was made in the scalp, six burr holes were drilled, a craniectomy was performed, and the dura was incised in an arcuate fashion. We attempted the interhemispheric approach and a retrosigmoid approach.
Results: It was confirmed that common neurosurgical approaches are achievable in this model. Furthermore, anatomical structures such as nerves and blood vessels were similar to those of humans. Moreover, the goat brain was similar in color and texture to that of humans.
Conclusion: Unlike a cadaver brain, in vivo brain requires hemostasis and careful dissection, which provides the surgeons a realistic experience of actual neurosurgery.
Keywords: Brain model, Cadaver dissection, Goats, Hemostasis, Neurosurgery
INTRODUCTION
At present, there are two ways to learn for novice and inexperienced neurosurgeon: By viewing operative videos and observing the surgical techniques of exceptional neurosurgeons, or by joining a cadaver dissection course and learning through hands-on practice.[
MATERIALS AND METHODS
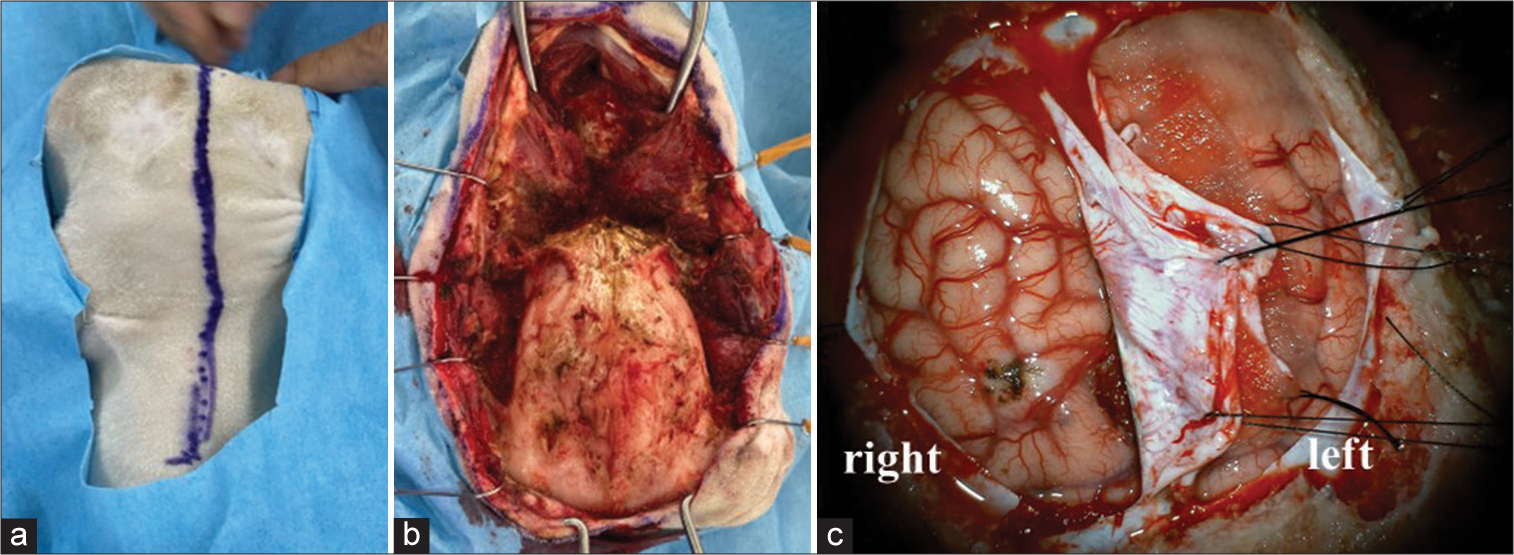
In this study, we used two female goats. They weighed 27.5 and 28.0 kg, respectively. Under general anesthesia, xylazine 0.4 mg/0.02/kg and atropine 0.5 mg were injected intramuscularly, and isoflurane 5% and O2 100% 1.5–2.0 ml/min were administered for anesthesia induction. The goat was intubated and ventilated to control breathing. Anesthesia was maintained with 2% isoflurane and 100% O2 at 1.5–2.0 ml/min. The goat was placed in the prone position and the head was patently fixed. A midline skin incision was made in the scalp [
RESULTS
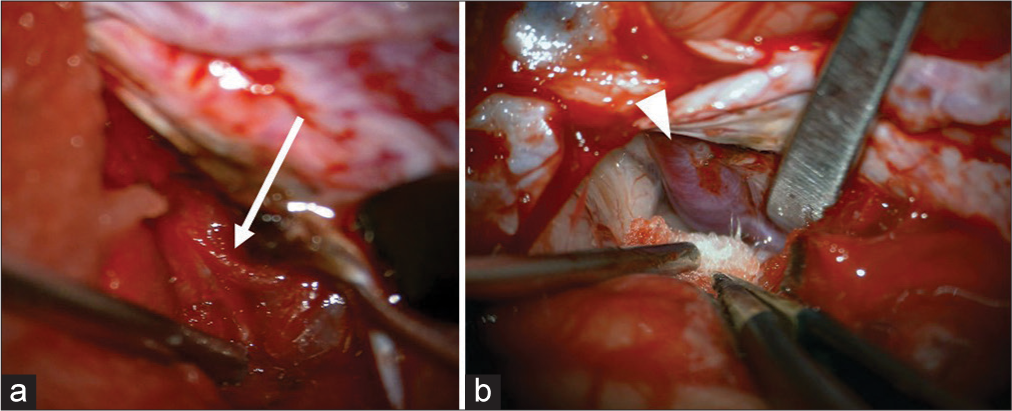
The dura mater was as thin as a sub-adult. The interhemispheric fissure showed the same morphology as that of a human. The bridging vein was fragile and it was then coagulated and cut. Pia matter, the texture of the brain, was similar to that of the human brain. The interhemispheric approach allowed us to reach the pericallosal artery [
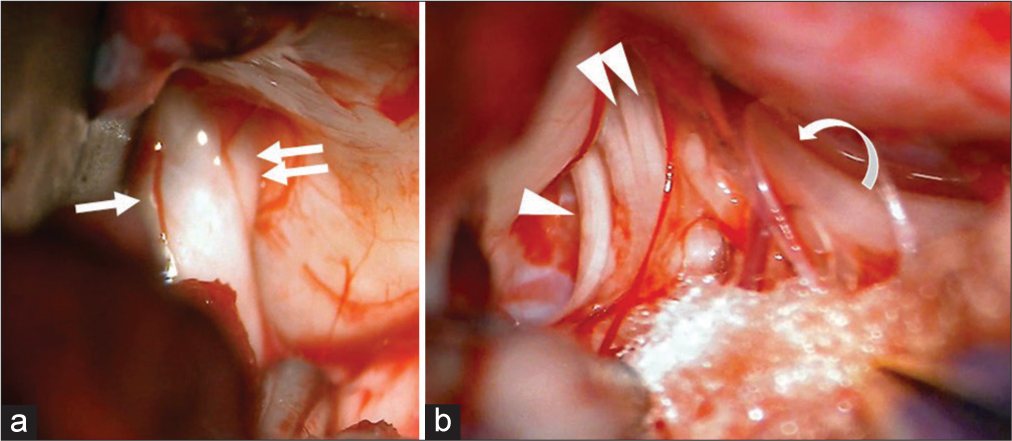
Figure 3:
Retrosigmoid approach (a) when the lateral cerebellum was compressed with a spatula, the facial nerve (arrow) and vestibulocochlear nerve (double arrows) entering the internal auditory canal were identified, (b) lower cranial nerve such as the glossopharyngeal nerve (arrow head), vagus nerves (double arrow heads), and accessory nerve (curbed arrow) were identified to be entering the jugular foramen.
DISCUSSION
There are reports of the use of bovine,[
This is the first report of neurosurgical training using a goat in vivo brain. Previously, only one report of a neurosurgical procedure training using goat ex vivo brain model has been published.[
It is difficult for young neurosurgeons to fully acquire neurosurgical skills.[
CONCLUSION
Neurosurgical technical training using in vivo brain models would be very useful for young neurosurgeons. Unlike a cadaver brain, in vivo brain requires hemostasis and careful dissection, which provides the surgeons a realistic experience of actual neurosurgery. The in vivo goat brain model has a texture and anatomical structure similar to humans; thus, it has high potential to be used for neurosurgical training.
Ethical approval
All procedures used in this research were approved by the Ethical Committee of International University of Health and Welfare.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to express our gratitude to Integra Japan K.K., led by Mr. Ito and Mr. Shibakawa, for their cooperation with goat brain neurosurgery training.
References
1. Aurich LA, Silva Junior LF, Monteiro FM, Ottoni AN, Jung GS, Ramina R. Microsurgical training model with nonliving swine head. Alternative for neurosurgical education. Acta Cir Bras. 2014. 29: 405-9
2. Cobb MI, Taekman JM, Zomorodi AR, Gonzalez LF, Turner DA. Simulation in neurosurgery-A brief review and commentary. World Neurosurg. 2016. 89: 583-6
3. Hamamcioglu MK, Hicdonmez T, Tiryaki M, Cobanoglu S. A laboratory training model in fresh cadaveric sheep brain for microneurosurgical dissection of cranial nerves in posterior fossa. Br J Neurosurg. 2008. 22: 769-71
4. Hanrahan J, Sideris M, Pasha T, Tsitsopoulos PP, Theodoulou I, Nicolaides M. Hands train the brain-what is the role of hand tremor and anxiety in undergraduate microsurgical skills?. Acta Neurochir (Wien). 2018. 160: 1673-9
5. Hicdonmez T, Hamamcioglu MK, Parsak T, Cukur Z, Cobanoglu S. A laboratory training model for interhemispherictranscallosal approach to the lateral ventricle. Neurosurg Rev. 2006. 29: 159-62
6. Hicdonmez T, Parsak T, Cobanoglu S. Simulation of surgery for craniosynostosis: A training model in a fresh cadaveric sheep cranium. J Neurosurg. 2006. 105: 150-2
7. Morosanu CO, Nicolae L, Moldovan R, Farcasanu AS, Filip GA, Florian IS. Neurosurgical cadaveric and in vivo large animal training models for cranial and spinal approaches and techniques–A systematic review of the current literature. Neurol Neurochir Pol. 2019. 53: 8-17
8. Sauleau P, Lapouble E, Val-Laillet D, Malbert CH. The pig model in brain imaging and neurosurgery. Animal. 2009. 3: 1138-51
9. Soubam P, Mishra S, Suri A, Dhingra R, Mochan S, Lalwani S. Standardization of the technique of silicon injection of human cadaveric heads for opacification of cerebral vasculature in Indian conditions. Neurol India. 2018. 66: 439-43
10. Turan Suslu H, Ceylan D, Tatarlı N, Hıcdonmez T, Seker A, Bayrı Y. Laboratory training in the retrosigmoid approach using cadaveric silicone injected cow brain. Br J Neurosurg. 2013. 27: 812-4