- Department of Neurosurgery Oncology, Latinoamerica Valerio Foundation, Weston, Florida, United States
- Vascular, Tumors and Functional Neurosurgery Service - Department of Neurosurgery, Guillermo Almenara Irigoyen National Hospital, Lima, Peru.
- Chair of the Functional Unit of the National Tumor Bank, National Institute of Neoplastic Diseases, Lima, Peru
Correspondence Address:
Jorge Zumaeta, Department of Neurosurgery Oncology, Latinoamerica Valerio Foundation, Weston, Florida, United States.
DOI:10.25259/SNI_202_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jorge Zumaeta1, Annel Murga2, Noe Santiago Rea1, Jose Daniel Flores-Sanchez2, Manuel Lazon2, Fernando Palacios Santos2, Sandro Casavilca Zambrano3, Immanuel Olarinde1, Jose Valerio1. Increase of primary intracranial sarcoma in children: Clinical manifestations, diagnosis, and management. 22-Nov-2024;15:426
How to cite this URL: Jorge Zumaeta1, Annel Murga2, Noe Santiago Rea1, Jose Daniel Flores-Sanchez2, Manuel Lazon2, Fernando Palacios Santos2, Sandro Casavilca Zambrano3, Immanuel Olarinde1, Jose Valerio1. Increase of primary intracranial sarcoma in children: Clinical manifestations, diagnosis, and management. 22-Nov-2024;15:426. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13247
Abstract
Background: Primary intracranial sarcomas (PISs) are very rare malignant tumors, and there is paucity of data on it, exclusively in patients
Methods: We retrospectively analyzed data in children diagnosed with PIS based on clinical presentation, imaging studies, and histopathology between January 2020 and December 2023.
Results: Twenty-five cases were identified. The median age was 5 years. There is slight female predominance (56%). On presentation, 68% of patients had features of intracranial hypertension (ICH), others had convulsions or motor deficits. There was radiologic evidence of cerebral hemorrhage in 80% of those with features of ICH and convulsion. All but one case had a supratentorial tumor. Emergency craniotomy was done in 84% of cases, and gross total resection (GTR) was achieved in the first surgery in 72% of cases. We used an adjuvant chemotherapyradiotherapy-chemotherapy (CTX-RT-CTX) regimen in 72% of cases, but 12% started this scheme 2 weeks after surgical resection. The cases followed up for more than a year that were managed with CTX-RT-CTX after GTR had a survival greater than a year, compared to the cases that received complementary treatment after 4 weeks.
Conclusion: PIS among children represents an infrequent pathology that, in the last years, its incidence has increased in Peru. The presence of intracerebral hemorrhage is a very suggestive finding of this diagnosis; therefore, emergent surgical management is an option before an irreversible ICH presents. Adjuvant treatment with the CTX-RT-CTX regimen started 2 weeks after GTR may improve survival in children with PIS.
Keywords: Cerebral hemorrhage, Children, Intracranial hypertension, Sarcoma, Neurosurgery
INTRODUCTION
Primary intracranial sarcomas (PISs) in children are rare and highly malignant. They are classified as mesenchymal non-meningothelial tumors.[
The true prevalence of PIS is unclear but varies from 0.1% to 4.3%.[
To the best of our knowledge, this manuscript is the second largest case series on pediatric PIS. Most published case series and case reports on PIS comprise only adults or a mixture of adults and children. There is a deficit of papers on PIS, exclusively in children.[
MATERIALS AND METHODS
At a tertiary hospital in Peru, we retrospectively collected data of patients <18 years old with immunohistochemical diagnosis of PIS from January 1, 2020, to December 31, 2023. We employed Maher et al. definition of PIS as sarcoma that originated in the brain from non-neuronal, non-glial, and non-reticular elements, with no previous evidence of systemic sarcoma and no sarcomatous transformation of a previously known benign tumor.[
RESULTS
Demography and presentation
None of the patients had any known risk factors. There were 14 females (56%) and 11 males (44%), from 2 to 17 years of age, with a median age of 5. Children under 10 years of age accounted for 84% of cases. On initial evaluation, most patients had signs and symptoms of intracranial hypertension (ICH) (68%), such as acute headache, nausea, and vomiting. Others presented with convulsions (20%) and motor deficits (12%). About 86% of all patients with ICH and convulsion had intracerebral hemorrhage, whereas only one in three patients with motor deficits had radiologic evidence of intracerebral hemorrhage.
Radiography
Preoperative CT scans showed all but one patient had supratentorial lesions. About 88% of the 24 supratentorial tumors were lobar, 8% were related to the falx cerebri in the midline, and 4% were in the basal ganglia. The tumors had a mixed pattern of contrast-enhancing solid portion accompanied by a cystic component. In all cases, the lesions were > 3 cm and compressing surrounding structures without evidence of leptomeningeal metastasis. In cases where brain magnetic resonance imaging (MRI) was performed, the lesions were hyperintense on T2, and fluid-attenuated inversion recovery showed flow voids and an irregular contrast enhancement. About 80% of cases had radiographic evidence of hemorrhage within the tumor and associated perilesional vasogenic edema. Three cases had radiographic evidence of intracerebral hemorrhage without a visible mass, followed by cerebral angiography, which was negative for arteriovenous malformation. Repeat scans, after neurologic deterioration, showed a tumor in the hemorrhagic zone, requiring emergency surgery.
Surgery
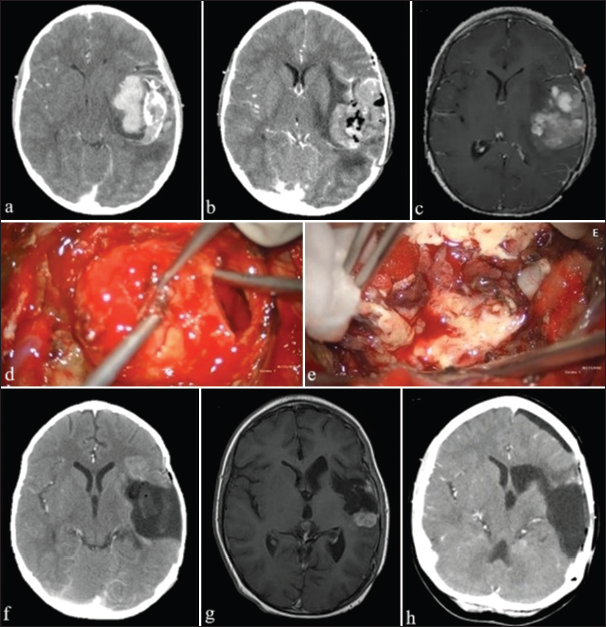
Most of the patients underwent emergency surgery for ICH and impaired consciousness. Almost half of the patients (48%) entered the OR with a Glasgow coma scale (GCS) ≤12. Case 12 had the lowest GCS of 4 associated with rebleeding [
Figure 2:
Brain scans of Case 12. (a and b) Initial computerized tomography (CT) scans show hemorrhagic brain tumor in the right temporal lobe; (c) repeat CT scan 3 days later shows expanding hemorrhage with midline shift, causing conscience deterioration (Glasgow comma scale of 4), warranting emergency surgery; (d) immediate postoperative CT scan confirms gross total resection and hemostasis; and (e) follow-up magnetic resonance imaging at 3 months shows no recurrence. The patient had an uneventful recovery for 2 years after surgery.
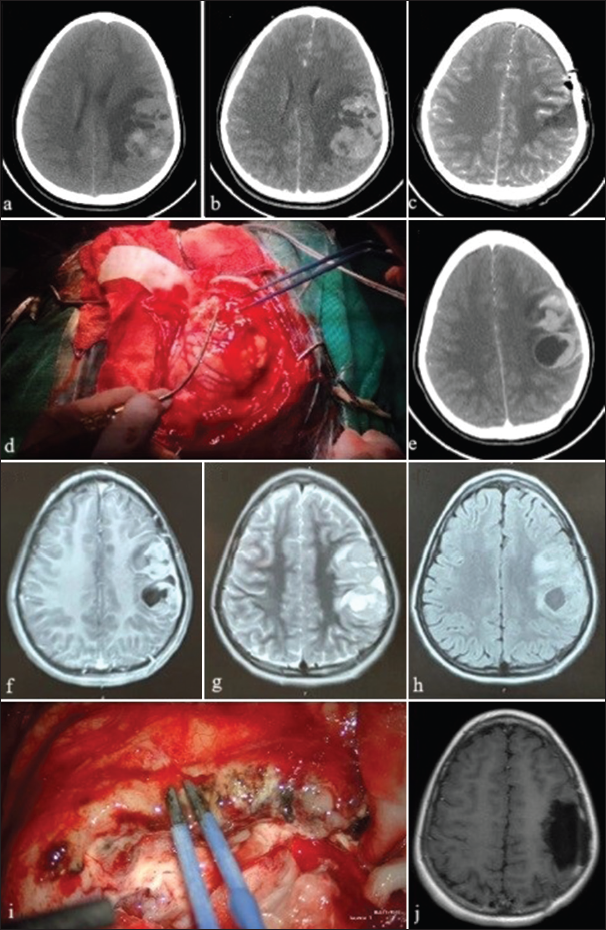
In 72% of the cases, a GTR was achieved in the first surgery. In 24% of cases, an STR was done in the first surgery, followed by a second GTR in three of those cases. In Case 10, PR was initially done due to profuse bleeding followed by GTR later [
Figure 3:
Case 10 brain scans and intraoperative images. (a) Hemorrhagic brain tumor in the left temporal and insular lobes on computerized tomography (CT) scan; (b) CT scan after emergency partial tumor resection and hemorrhage control; (c) preoperative magnetic resonance imaging (MRI) before second surgery; (d) exposed vascularized tumor during second surgery; (e) surgical bed after gross total resection (GTR) of tumor during second surgery; (f) postoperative CT scan confirms GTR; (g) MRI scan 18-months-after second surgery shows recurrent lesions; and (h) CT scan after GTR of recurrent lesions.
In some cases, the use of fluorescence aided GTR. Grossly, the tumors are pearly or light brown, soft, friable, highly vascularized, necrotic, and hemorrhagic. We identified several friable vessels that are difficult to control. Most of the lesions in our series are infiltrating the cerebral parenchyma and the meninges. Only a few had a well-defined plane of dissection. The preferred surgical technique was circumferential dissection with devascularization followed by “en bloc” tumor removal. In addition, we resected infiltrated meningeal tissue (dura mater or the falx cerebri), followed by meningeal repair with periosteum or fascia lata.
Pathology
The pathology department reported two histologic features. Fusiform spindle cells with large hyperchromatic nuclei embedded in abundant pinkish stroma are described as fusocellular sarcoma and highly vascularized poorly differentiated pleomorphic cells consistent with high-grade sarcoma. No molecular diagnosis was done to determine specific subtypes.
Figure 4:
Histopathology of primary intracranial sarcoma in Case 1. (a) Intracranial sarcoma showing pleomorphic features and prominent vasculature. Hematoxylin and eosin (H&E) stain ×20 objective, 200 augments; (b) cytoplasmic hyaline globules in pleomorphic sarcoma. H&E stain ×40 objective, 400 augments; (c) atypical multipolar mitosis and marked nuclear anaplasia with the presence of cytoplasmic hyaline globules. H&E stain ×40 objective, 400 augments; and (d) intracranial sarcoma showing prominent vasculature and tumoral cells cytoplasmic vacuolization. H&E stain ×20 objective, 200 augments.
Immunohistochemical studies in all patients were negative for glial fibrillary acidic protein (GFAP) but positive for vimentin and P53. Eighty percent had over 70% Ki-67 levels. Three cases initially diagnosed as giant cell glioblastoma, gliosarcoma, and hemangiopericytoma were changed to PIS based on immunohistochemical results.
In some of our cases, it was possible to identify focal chondroid differentiation, focal rhabdomyoblastic differentiation, and focal leiomyomatous differentiation, highlighting the rhabdomyoblastic type in the patients who presented intracerebral hemorrhage.
Adjuvant therapies
CTX before and after RT was administered to 68% of patients. The CTX regimen consisted of ifosphamide, cisplatin, and etoposide. About 60% of cases received CTX during the 1st month after surgery, while 12% received CTX 2 weeks after surgery. We treated only one case with radiosurgery and bevacizumab. The delay in adjuvant therapy could be attributed to late pathology reports, high patient volume, inconsistent follow-up, or poor accessibility to the hospital from rural areas.
Outcome
Eighteen patients were alive at the end of the study. Patients who did not receive CTX and RT on time had a greater recurrence rate and decreased overall survival [
Figure 5:
Brain scans and intraoperative images of Case 1. (a and b) Computerized tomography (CT) scans of left frontoparietal hemorrhagic tumor with mild midline shift; (c) postoperative CT scan confirms gross total resection; (d) exposed tumor during first surgery; (e) hyperintense lesions at site of initial surgery suggestive of recurrence 1 month after first surgery; (f-h) magnetic resonance imaging (MRI) scan of recurrent lesions at surgical site; (i) fluorescein-guided resection of recurrent lesions during second surgery; and (j) postoperative MRI confirms successful resection of recurrent lesions.
Other representative Cases, 2 and 15, are shown in
Figure 6:
Scans and intraoperative image of Case 2. (a-c) Axial scans show hemorrhagic brain tumor with falx cerebri infiltration; (d and e) sagittal scans show midline hyperintense irregular mass attached to the falx cerebri; (f) intraoperative image of craniotomy and tumor resection through an interhemispheric approach; and (g) postoperative scan confirms successful gross total resection.
Figure 7:
Scans and intraoperative image of Case 15. (a) Coronal cranial magnetic resonance imaging (MRI) after decompressive craniotomy for intracerebral hemorrhage show giant brain tumor in the left cerebral hemisphere; (b) sagittal cranial MRI shows a heterogenous mass with surrounding edema and diffuse intracerebral hemorrhage; (c and d) axial head computerized tomography (CT) scan without contrast and with contrast showing a mass in the left cerebral hemisphere, mild hemorrhage, and brain herniation through craniotomy; (e) intraoperative image during subtotal tumor resection, duraplasty with bovine pericardium, and coverage of cranial defect with titanium mesh; and (f) postoperative axial CT scan showing residual tumor with hemostatic control.
DISCUSSION
The prevalence of PIS ranges from 0.1% to 4.3%.[
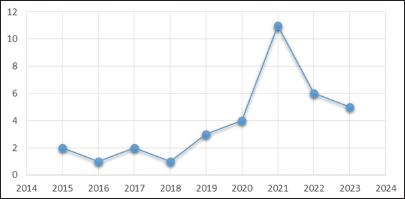
We report 25 cases at a single hospital over 4 consecutive years (2020–2023), with a peak incidence of 11 cases in 2021. The incidence of primary sarcomas in children in Peru is very high compared to other countries.[
Nonspecific imaging features of PIS make diagnosis difficult. Most have a mixed solid-cystic appearance with perilesional edema and associated intracerebral hemorrhage. The solid component is enhanced by contrast due to hypervascularity, as also demonstrated in other studies.[
In most of our cases, tumors were supratentorial and located proximal to the meninges, like other series.[
Compared to adults, sarcomas in children tend to be either undifferentiated or differentiate into myocytes[
The mainstay of treatment is surgery, especially in patients with ICH. Most studies propose radical surgical resection [
We achieved GTR in most cases, but only 12% received adjuvant therapies 2 weeks after surgery. We observed better disease control and lower recurrence rate in those who got adjuvant therapy 2 weeks after surgery compared to those who did not. In addition, all patients who got adjuvant therapy 2 weeks after surgery were alive for 1 year, emphasizing the importance of early adjuvant therapy. After surgery, Case 6 attained adequate disease control with radiosurgery and bevacizumab. The benefits of combination radiosurgery and bevacizumab require further investigation.[
Although the overall prognosis of PIS is poor, as observed in our study and corroborated by other papers, GTR followed by early CTX and RT might improve prognosis.[
Limitations and future directions
Despite the high incidence of PIS in children in Peru, this study should be cautiously generalized since it was carried out at a single center. Despite our case series being larger than other series, it is small to perform a quantitative statistical analysis. Therefore, we made a primarily descriptive analysis of the results. The number of surgical resections may modify or confound the true impact of early adjuvant therapy on patient survival. In addition, the short follow-up and study duration preclude concluding the long-term effects of treatment modalities on prognosis. Larger case series and prospective studies with genetic analysis are required to investigate the differences in survival among different therapeutic subgroups observed in this study.
CONCLUSION
Even though Peru is a country with a high prevalence of PIS in children, there was an increased incidence between 2020 and 2023 in tertiary hospitals for still unknown reasons. Patients often present with signs and symptoms of increased intracranial pressure due to intracerebral hemorrhage. Most are supratentorial cortical tumors located beneath the meninges. Imaging studies typically show intracerebral hemorrhage irregular contrast enhancement with perilesional edema. Immunohistochemical staining confirms the diagnosis. Although there is still no consensus and established protocol for the management of PIS, safe maximum resection followed by CTX and focal RT may offer the best long-term survival. Repeat surgery can be done for recurrent local disease. We devote more effort to fully understanding the genetics of PIS because molecular patterns could help personalize adjuvant therapy.
Ethical approval
The Institutional Review Board approval is taken, approval no is : NIT 753-2024-484 / Project 103-2024.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
We would like our sincere thanks to the entire team at Vascular, Tumors and Functional Neurosurgery Service, Neurosurgery Department, Guillermo Almenara Irigoyen National Hospital, for their unwavering dedication and expertise in treating patient with brain tumors. Their collaborative efforts and exceptional care have greatly contributed to the presentation of this work.
We would also like to express our gratitude to the Latinoamerica Valerio Foundation, particularly to CFO Dr. Silvana Valerio, Director of research and innovation Dr. Andres Alvarez Pinzon, and Dr. Maria P. Fernandez for their valuable collaboration and support in the completion of this paper.
References
1. Al-Gahtany M, Shroff M, Bouffet E, Dirks P, Drake J, Humphreys R. Primary central nervous system sarcomas in children: Clinical, radiological, and pathological features. Childs Nerv Syst. 2003. 19: 808-17
2. Benesch M, von Bueren AO, Dantonello T, von Hoff K, Pietsch T, Leuschner I. Primary intracranial soft tissue sarcoma in children and adolescents: A cooperative analysis of the European CWS and HIT study groups. J Neurooncol. 2013. 111: 337-45
3. Diaz Coronado RY, Mynarek M, Koelsche C, Mora Alferez P, Casavilca Zambrano S, Wachtel Aptowitzer A. Primary central nervous system sarcoma with DICER1 mutation-treatment results of a novel molecular entity in pediatric Peruvian patients. Cancer. 2022. 128: 697-707
4. El-Ayadi M, Ansari M, Kühnöl C, Bendel A, Sturm D, Pietsch T. EPID-18. Noonan syndrome can be associated with high grade glioma, a report of two cases. Neuro Oncol. 2017. 19: vi72
5. Flannery T, Kano H, Niranjan A, Monaco EA, Flickinger JC, Kofler J. Gamma knife radiosurgery as a therapeutic strategy for intracranial sarcomatous metastases. Int J Radiat Oncol Biol Phys. 2010. 76: 513-9
6. Kamihara J, Paulson V, Breen MA, Laetsch TW, Rakheja D, Shulman DS. DICER1-associated central nervous system sarcoma in children: Comprehensive clinicopathologic and genetic analysis of a newly described rare tumor. Mod Pathol. 2020. 33: 1910-21
7. Koelsche C, Mynarek M, Schrimpf D, Bertero L, Serrano J, Sahm F. Primary intracranial spindle cell sarcoma with rhabdomyosarcoma-like features share a highly distinct methylation profile and DICER1 mutations. Acta Neuropathol. 2018. 136: 327-37
8. Lafay-Cousin L, Lindzon G, Taylor MD, Hader W, Hawkins C, Nordal R. Successful treatment of primary intracranial sarcoma with the ICE chemotherapy regimen and focal radiation in children. J Neurosurg Pediatr. 2016. 17: 298-302
9. Lazarte-Rantes C, Pillaca-Cruzado O, Baca-Hinojosa N, Mamani W, Lee-Diaz J, Ugas-Charcape CF. MRI findings of primary intracranial sarcomas in children. Pediatr Radiol. 2023. 53: 1698-703
10. Lodi M, Boccuto L, Carai A, Cacchione A, Miele E, Colafati GS. Low-grade gliomas in patients with noonan syndrome: Case-based review of the literature. Diagnostics (Basel). 2020. 10: 582
11. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021. 23: 1231-51
12. Lovaton-Espadin R, Alaba-Garcia W. EPID-25. Six consecutive cases of primary intracranial sarcoma in children in two private institutions from lima during COVID-19 pandemic. Neuro Oncol. 2023. 25: v121
13. Maher OM, Khatua S, Mukherjee D, Olar A, Lazar A, Luthra R. Primary intracranial soft tissue sarcomas in children, adolescents, and young adults: Single institution experience and review of the literature. J Neurooncol. 2016. 127: 155-63
14. McWilliams GD, SantaCruz K, Hart B, Clericuzio C. Occurrence of DNET and other brain tumors in Noonan syndrome warrants caution with growth hormone therapy. Am J Med Genet A. 2016. 170A: 195-201
15. Melgar-Granados F, Segundo CH. Primary intracranial spindle cell sarcoma with features similar to rhabdomyosarcoma in pediatric patients: Cases series 2020-2021. Intercien Méd. 2023. 13: 50-8
16. Niwa J, Hashi K, Minase T. Radiation induced intracranial leiomyosarcoma: Its histopathological features. Acta Neurochir (Wien). 1996. 138: 1470-1
17. Rueda-Franco F, López-Corella E. Sarcomas in the central nervous system of children. Pediatr Neurosurg. 1995. 22: 49-56
18. Sakaguchi M, Nakano Y, Honda-Kitahara M, Kinoshita M, Tanaka S, Oishi M. Two cases of primary supratentorial intracranial rhabdomyosarcoma with DICER1 mutation which may belong to a “spindle cell sarcoma with rhabdomyosarcoma-like feature, DICER1 mutant”. Brain Tumor Pathol. 2019. 36: 174-82
19. Yang G, Yuan Z, Ahmed K, Welsh EA, Fulp WJ, Gonzalez RJ. Genomic identification of sarcoma radiosensitivity and the clinical implications for radiation dose personalization. Transl Oncol. 2021. 14: 101165