- Department of Neurosurgery, Houston Methodist Neurological Institute, Houston, Texas, United States
- Department of Pathology, Houston Methodist Hospital, Houston, Texas, United States
- Department of Internal Medicine, Houston Methodist Hospital, Houston, Texas, United States
- Kenneth R. Peak Presidential Distinguished Chair Vice Chairman and Residency Program Director Department of Neurosurgery Director, Kenneth R. Peak Brain and Pituitary Tumor Treatment Center Professor of Neurosurgery, The Houston Methodist Research Institute, Weill Cornell Medical College and Texas A&M Medical School, Houston, Texas, United States.
Correspondence Address:
Dr. Amanda Vilate Jenson, Department of Neurosurgery, Houston Methodist Neurological Institute, Houston, Texas, United States.
DOI:10.25259/SNI_507_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Amanda Vilate Jenson1, Daniel G. Taylor2, Alberto Ayala2, Robert Evan Jackson3, David S. Baskin4. Indolent multicentric chordoma – A previously undescribed entity: A Case report and literature review. 12-Aug-2022;13:348
How to cite this URL: Amanda Vilate Jenson1, Daniel G. Taylor2, Alberto Ayala2, Robert Evan Jackson3, David S. Baskin4. Indolent multicentric chordoma – A previously undescribed entity: A Case report and literature review. 12-Aug-2022;13:348. Available from: https://surgicalneurologyint.com/surgicalint-articles/11798/
Abstract
Background: Chordomas are rare neuraxial tumors arising from remnants of primitive notochord. They are generally slow-growing malignant neoplasms. Only four adult cases of multicentric chordomas have been reported, all with aggressive and rapid growth. Here, we present an unusual case of indolent multicentric chordomas involving cervical and thoracic spine, sacrum, and calvarium.
Case Description: A 60-year-old male was found to have multiple lesions throughout his neuroaxis incidentally on workup for colitis. A needle biopsy documented the diagnosis of chordoma. This has been followed for more than 4 years with no progression.
Conclusion: We present the first reported case of indolent multicentric chordomas. Due to the extreme rarity of indolent multicentric chordomas, close follow-up is needed and recommended.
Keywords: Chordoma, Indolent, Multicentric
INTRODUCTION
Chordomas are rare neuraxial tumors arising from remnants of primitive notochord. Chordomas generally are slow-growing malignant neoplasms, usually with aggressive clinical behavior with invasive bony erosion.[
CLINICAL PRESENTATION
A 60-year-old man with newly diagnosed colitis presented for a computed tomography (CT) scan of the abdomen for assessment. This incidentally demonstrated osteolytic lesions of the spine. CT and magnetic resonance imaging (MRI) scans of the entire neuroaxis were performed showing multiple lesions involving the cervical/thoracic spine, sacrum, and left parietal scalp/ bone,
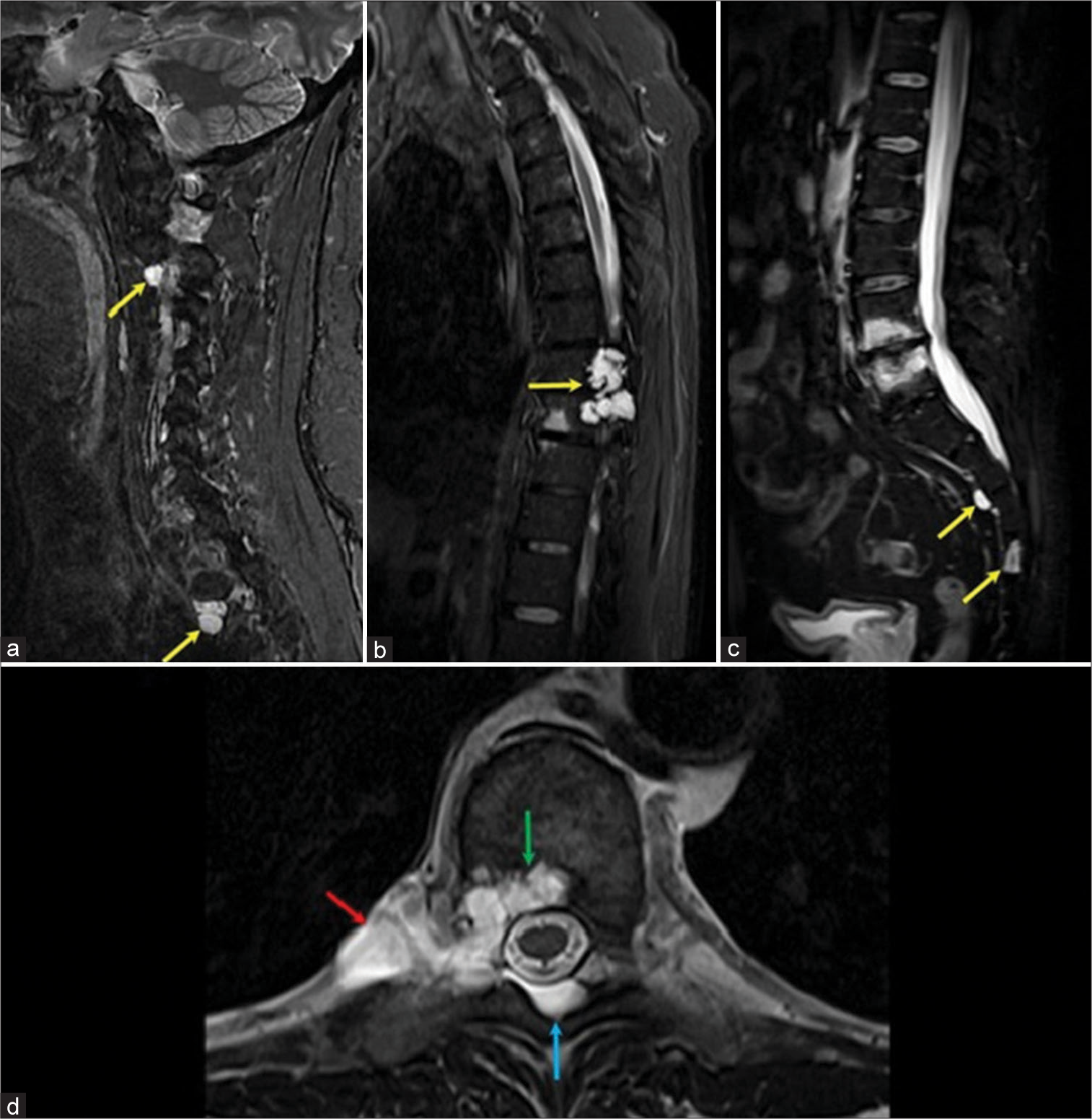
Figure 1:
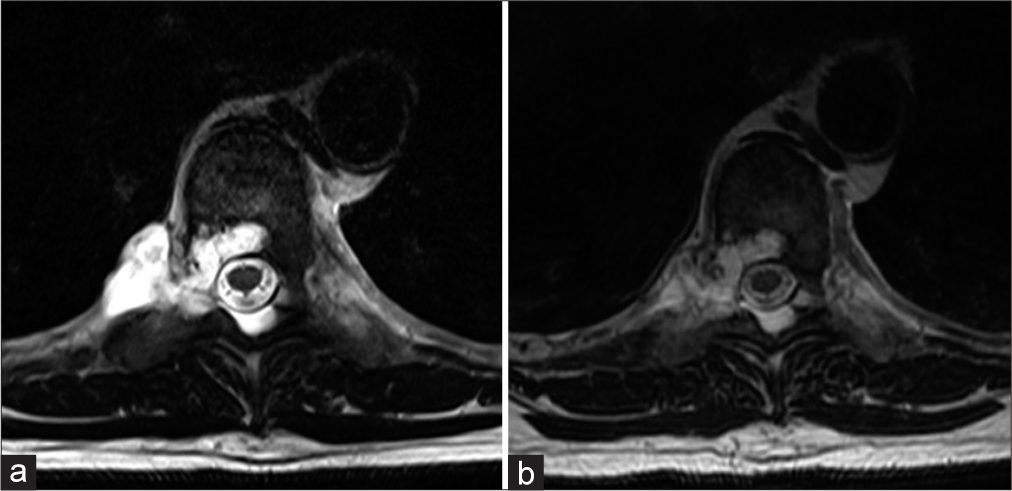
(a) STIR magnetic resonance imaging (MRI) parasagittal view of the cervical spine. (b) STIR MRI mid-sagittal view of thoracic spine. (c) MRI STIR mid-sagittal view of lumbar/sacral spine. (a-c) shows multiple mildly enhancing spinal lesions with very high T2 signal best seen on STIR (yellow arrows) at C2-3, C3-4, C5-6, T2-3, T3-4, T8-9, S2-3, and S4 levels. These lesions have variable bone and soft tissue involvement. (d) T2 axial image of the dominant lesion at T8-9 level, showing a lobulated T2-hyperintense lesion with well-defined margins involving bone (green arrow) with paraspinal (red arrow) and epidural (blue arrow) extension.
The patient sought a third opinion 2 years later after developing mild left calf and foot pain. A needle biopsy of the dominant lesion at the T8-9 level was performed in July 2020. Histological analysis of the paraspinal mass biopsy was consistent with chordoma. The specimen contained nests and lobules of cells with rounded nuclei and abundant eosinophilic vacuolated cytoplasm focally, with myxoid material seen in the background, [
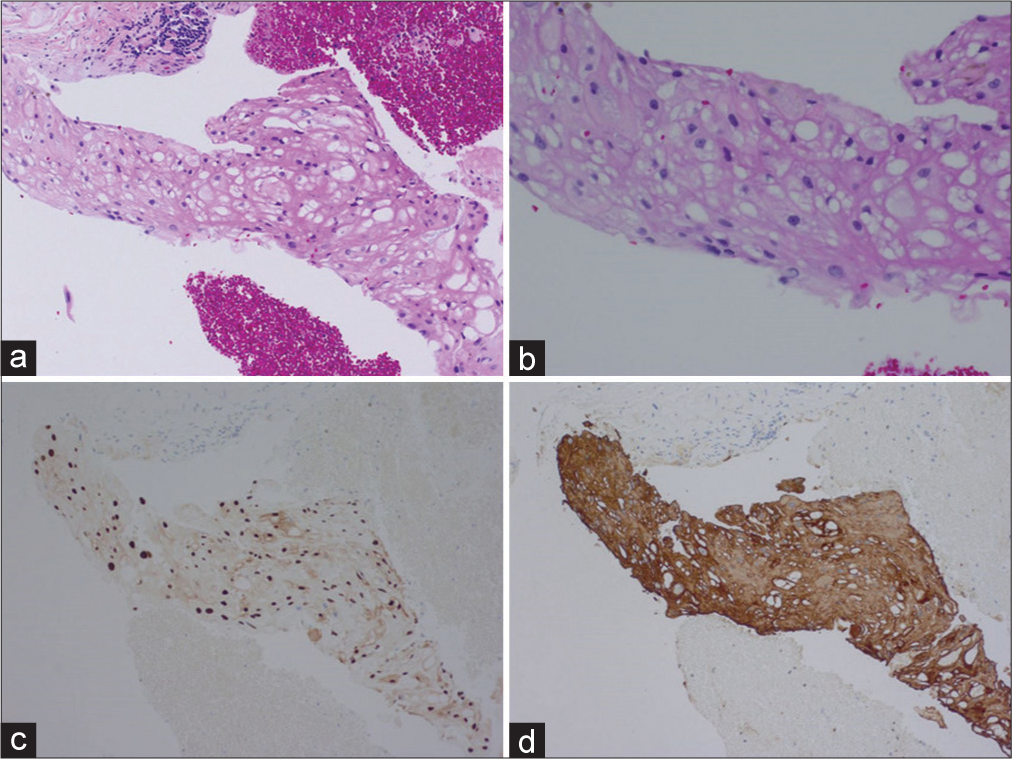
Figure 2:
(a and b) H&E sections from T8-9 vertebral body mass demonstrating enlarged multivacuolated and physaliphorous cells with round to ovoid nuclei. Immunohistochemical staining demonstrates strong nuclear positivity for brachyury (c) and strong cytoplasmic staining for AE 1/3 (d) These pathologic findings are classic for chordoma.
He then presented at our facility for another opinion about optimal management of his condition in November, 2020. He underwent updated MRI scans of his entire neuroaxis to see if the lesions grew. The MRI scans showed no change in all locations.
Continued serial observation without further intervention was recommended. He has since had repeat imaging in November 2021 and May 2022 that, again, show no change in all of the lesions.
DISCUSSION
The differential diagnosis for multiple vertebral body lytic and T2 hyperintense lesions includes metastasis, multiple myeloma, or plasmacytoma. Periosteal or exophytic lesions, which may be in atypical chordoma locations, can be mistaken for benign cysts. After the pathology slides from the biopsy were sent to our facility for review by our pathologist, the diagnosis was confirmed as multicentric chordoma.
Ecchordosis physaliphora was taken into consideration. Both chordoma and ecchordosis physaliphora are notochordal remnants with the same characteristic pathological features. However, ecchordosis physaliphora is usually in a clival location and does not enhance with gadolinium infused MRIs.[
Little is known about multicentric chordomas, as there have only been four previous cases in the literature.[
The literature on multicentric chordomas is scant[
Chordomas are remnants of notochord and are made up of epithelioid cells, with a dual epithelial-mesenchymal differentiation, immersed in a myxoid stroma. Chordomas are typically positive for EMA, cytokeratin, S100, and brachyury. Brachyury is a transcription factor involved in notochordal differentiation; its positivity is useful in the differential diagnosis from chondrosarcoma[
The gold standard of treatment for chordoma is surgery with wide margins; however, more than 50% can recur after treatment.[
Chordomas are relatively radioresistant, requiring high-dose radiation therapy. Since chordomas are located around critical neuronal structures, highly conformal proton beam radiation is used. Resection, combined with chemoradiation increases the overall 5-year survival from 50% to 65%.[
CONCLUSION
Despite the advancements in treatment for chordomas, little is known about multicentric chordomas, especially ones that are stable. Indolent lesions such as in our patient, with incidentally found multicentric chordomas, with the evidence of no progression for over 4 years have not been reported. We plan close follow-up, with his most recent stable imaging from May 2022, with serial imaging over the next 5 years. The entity of indolent multicentric chordoma is novel, and to the best of our knowledge, ours is the first reported case in the literature.
Verbal consent was obtained from the patient.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
We are grateful to the many patients and families who have helped us with our research. This work was supported by the Donna and Kenneth R. Peak Foundation, The Kenneth R. Peak Brain and Pituitary Center at Houston Methodist Hospital, The Taub Foundation, The Blanche Green Estate Fund of the Pauline Sterne Wolff Memorial Foundation, The John S. Dunn Foundation, Kelly Kicking Cancer, The Marilee A. and Gary M. Schwarz Foundation, The Houston Methodist Hospital Foundation, and the Verelan Foundation.
Conflicts of interest
There are no conflicts of interest.
References
1. Anderson WB, Meyers HI. Multicentric chordoma. Report of a case. Cancer. 1968. 21: 126-8
2. Di Maio S, Rostomily R, Sekhar LN. Current surgical outcomes for cranial base chordomas: Cohort study of 95 patients. Neurosurgery. 2012. 70: 1355-60
3. Fujiwara T, Tsuda Y, Stevenson J, Parry M, Jeys L. Sacral chordoma: Do the width of surgical margin and the use of photon/proton radiotherapy affect local disease control?. Int Orthop. 2020. 44: 381-9
4. George B, Bresson D, Herman P, Froelich S. Chordomas: A review. Neurosurg Clin N Am. 2015. 26: 437-52
5. Grossbach A, Baimeedi P, McDonald W, Bergman T. Multicentric chordoma: A case report and review of the literature. Neurosurgery. 2011. 69: E1327-32
6. Heery CR, Palena C, McMahon S, Donahue RN, Lepone LM, Grenga I. Phase I study of a poxviral TRICOM-based vaccine directed against the transcription factor brachyury. Clin Cancer Res. 2017. 23: 6833-45
7. Hindi N, Casali PG, Morosi C, Messina A, Palassini E, Pilotti S. Imatinib in advanced chordoma: A retrospective case series analysis. Eur J Cancer. 2015. 51: 2609-14
8. Jones JR, Huttner A, Malhotra A. Multicentric chordoma: An uncommon and incompletely understood presentation. Clin Neuroradiol. 2018. 28: 283-8
9. Lagman C, Varshneya K, Sarmiento JM, Turtz AR, Chitale RV. Proposed diagnostic criteria, classification schema, and review of literature of notochord-derived ecchordosis physaliphora. Cureus. 2016. 8: e547
10. Lim JJ, Kim SH, Cho KH, Yoon DH, Kim SH. Chordomas involving multiple neuraxial bones. J Korean Neurosurg Soc. 2009. 45: 35-8
11. McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: Incidence and survival patterns in the United States 1973-1995. Cancer Causes Control. 2001. 12: 1-11
12. Miettinen M, Wang Z, Lasota J, Heery C, Schlom J, Palena C. Nuclear brachyury expression is consistent in chordoma, common in germ cell tumors and small cell carcinomas, and rare in other carcinomas and sarcomas: An immunohistochemical study of 5229 cases. Am J Surg Pathol. 2015. 39: 1305-12
13. Papagelopoulos PJ, Mavrogenis AF, Galanis EC, Savvidou OD, Boscainos PJ, Katonis PG. Chordoma of the spine: Clinicopathological features, diagnosis, and treatment. Orthopedics. 2004. 27: 1256-63
14. Sahyouni R, Goshtasbi K, Mahmoodi A, Chen JW. A historical recount of chordoma. J Neurosurg Spine. 2018. 28: 422-8
15. Sebro R, DeLaney TF, Hornicek F, Schwab J, Choy E, Nielsen GP. Frequency and risk factors for additional lesions in the axial spine in subjects with chordoma: Indications for screening. Spine (Phila Pa 1976). 2017. 42: E37-40
16. Stacchiotti S, Casali PG. Systemic therapy options for unresectable and metastatic chordomas. Curr Oncol Rep. 2011. 13: 323-30
17. Stacchiotti S, Morosi C, Lo Vullo S, Casale A, Palassini E, Frezza AM. Imatinib and everolimus in patients with progressing advanced chordoma: A phase 2 clinical study. Cancer. 2018. 124: 4056-63