- Department of Neurosurgery, Kanazawa University, Kanazawa, Ishikawa, Japan
- Department of Neurosurgery, Kanazawa Medical University, Kahoku, Ishikawa, Japan.
Correspondence Address:
Yasuo Sasagawa, Department of Neurosurgery, Kanazawa University, Kanazawa, Ishikawa, Japan.
DOI:10.25259/SNI_909_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Munehiro Demura1, Yasuo Sasagawa1, Yasuhiko Hayashi2, Osamu Tachibana2, Mitsutoshi Nakada1. Inferior temporal quadrantanopia associated with pituitary adenomas and a potential mechanism of excessive optic nerve bending. 01-Mar-2024;15:70
How to cite this URL: Munehiro Demura1, Yasuo Sasagawa1, Yasuhiko Hayashi2, Osamu Tachibana2, Mitsutoshi Nakada1. Inferior temporal quadrantanopia associated with pituitary adenomas and a potential mechanism of excessive optic nerve bending. 01-Mar-2024;15:70. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12773
Abstract
Background: Pituitary adenomas show typical visual field defects that begin superiorly and progress inferiorly. The cause of atypical visual field defects that start inferiorly remains unclear. This study aimed to understand this phenomenon using magnetic resonance imaging (MRI).
Methods: A total of 220 patients with pituitary adenomas underwent a visual field assessment of both eyes. Preoperative visual fields were assessed and classified into two types: superior quadrantanopia (typical) and inferior quadrantanopia (atypical). Several parameters related to tumor characteristics and optic nerve compression were evaluated using MRI.
Results: Of the 440 eyes examined, 174 (39.5%) had visual field defects. Of these, 28 (16.1%) had typical and 11 (6.3%) had atypical visual field defects. Patient age, tumor size, degree of cavernous sinus invasion, tumor pathology, and intratumor bleeding were similar between the two groups. The angle formed by the optic nerve in the optic canal and in the intracranial subarachnoid space at the exit of the optic canal (degree of optic nerve bending) was significantly larger in the atypical group than in the typical group (42.6° vs. 23.9°, P = 0.046).
Conclusion: In some pituitary adenomas, visual field defects begin inferiorly. This may be caused by optic nerve compression on the superior surface by the bony margin of the optic canal exit. Therefore, pituitary adenomas should be considered in patients with atypical visual field defects.
Keywords: Magnetic resonance image, Optic nerve, Pituitary adenoma, Visual field defects
INTRODUCTION
Visual field defects with pituitary adenomas typically present as bilateral hemianopia and are thought to be caused by upward compression of the optic chiasm by the tumor, resulting in the progression of the visual field defects from the superior to anterior position.[
MATERIALS AND METHODS
Study population
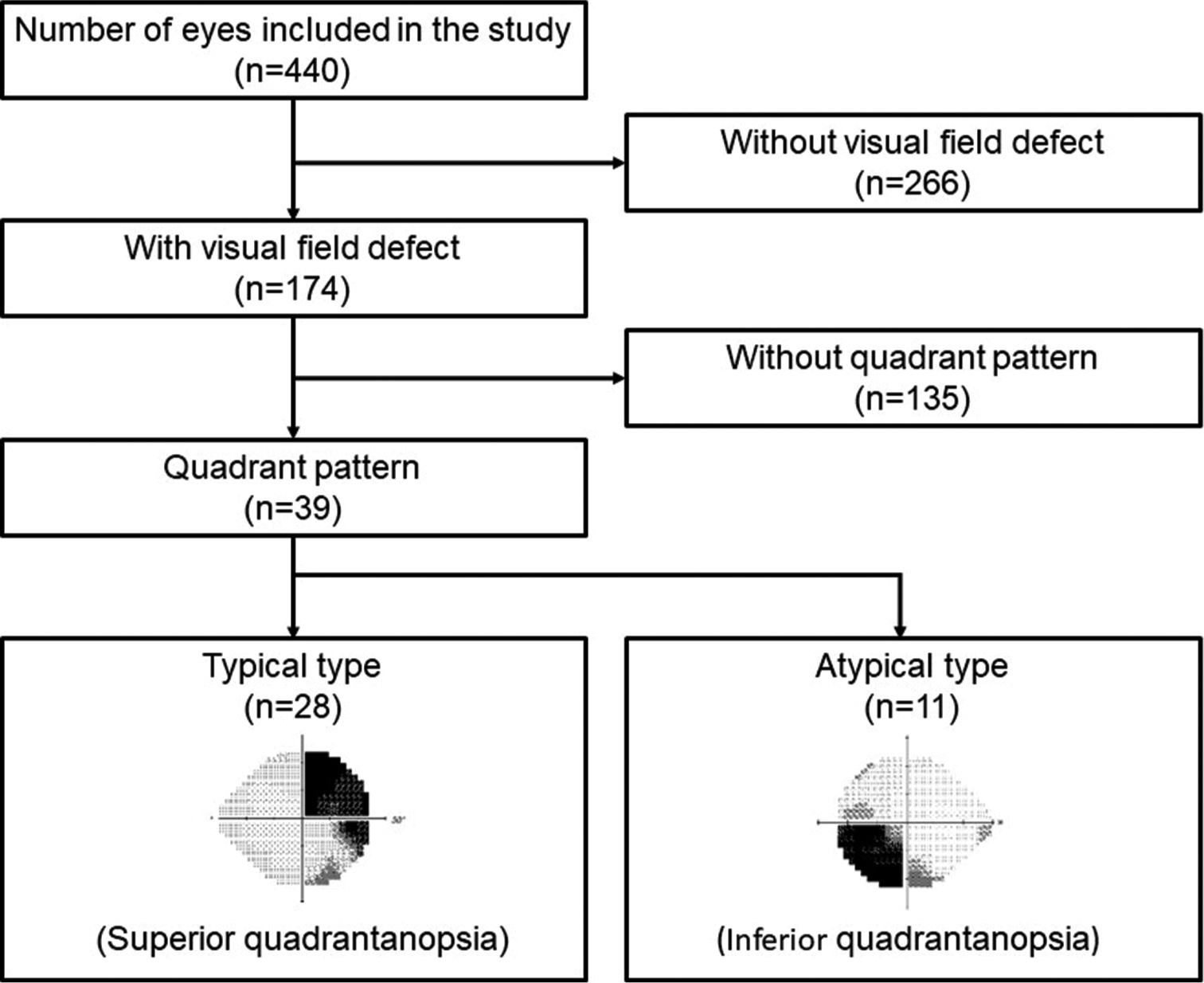
This study is a retrospective review of 220 patients who underwent transnasal transsphenoidal surgery for pituitary adenomas between 2001 and 2019 at Kanazawa University Hospital. The Institutional Review Board has approved the study protocol (No. 2014032) and waived the need for informed consent. Patients who had a history of pituitary surgery or radiation therapy, ophthalmic diseases such as glaucoma, or previous craniotomy were excluded from our study. Patient history, including age, sex, tumor histology, radiologic findings, medical history, and visual function impairment, were obtained from medical records. A flowchart depicting inclusion or exclusion criteria is shown in
Ophthalmologic evaluation
Ophthalmologic examinations were performed by experienced ophthalmologists who evaluated the preoperative and postoperative visual fields. Radiologic findings of the patients were not disclosed to the ophthalmologists. Postoperative evaluations were performed within a week after surgery. Visual fields were assessed using a Humphrey field analyzer and kinetic Goldmann perimetry. For qualitative assessment, visual field defects were classified into two types based on the pattern of loss in each eye. Typical types of defects included only the superior quadrant, while the atypical type of defects included the inferior quadrant.
Radiologic evaluation
Preoperative MRI images of the pituitary region were obtained for all patients using a 3-T scanner (MAGNETOM Trio; Siemens, Munich, Germany; or Signa Excite HDx; GE Healthcare, Tokyo, Japan). Pre-enhanced T1- and T2-weighted images and post-enhanced T1-weighted images were obtained for all patients in the sagittal and coronal planes with a 1.5 mm slice thickness. Tumor measurements were defined as follows: maximum craniocaudal diameter was defined as the tumor height; vertical length from the anterior skull base to the top of the tumor was defined as the tumor’s top-anterior skull base distance; and cavernous sinus invasion of the tumor was evaluated based on the Knosp grade on coronal images.[
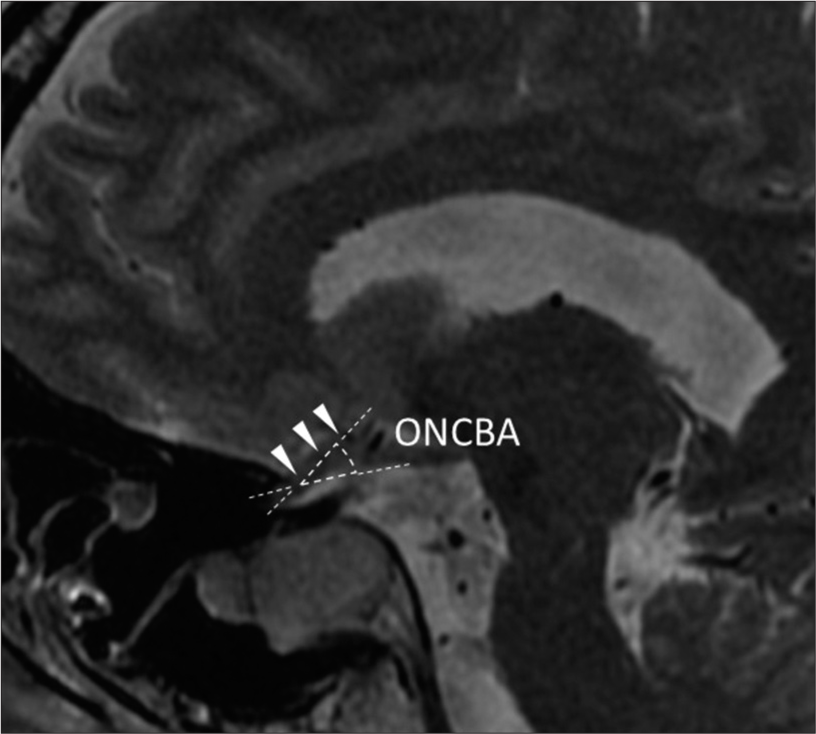
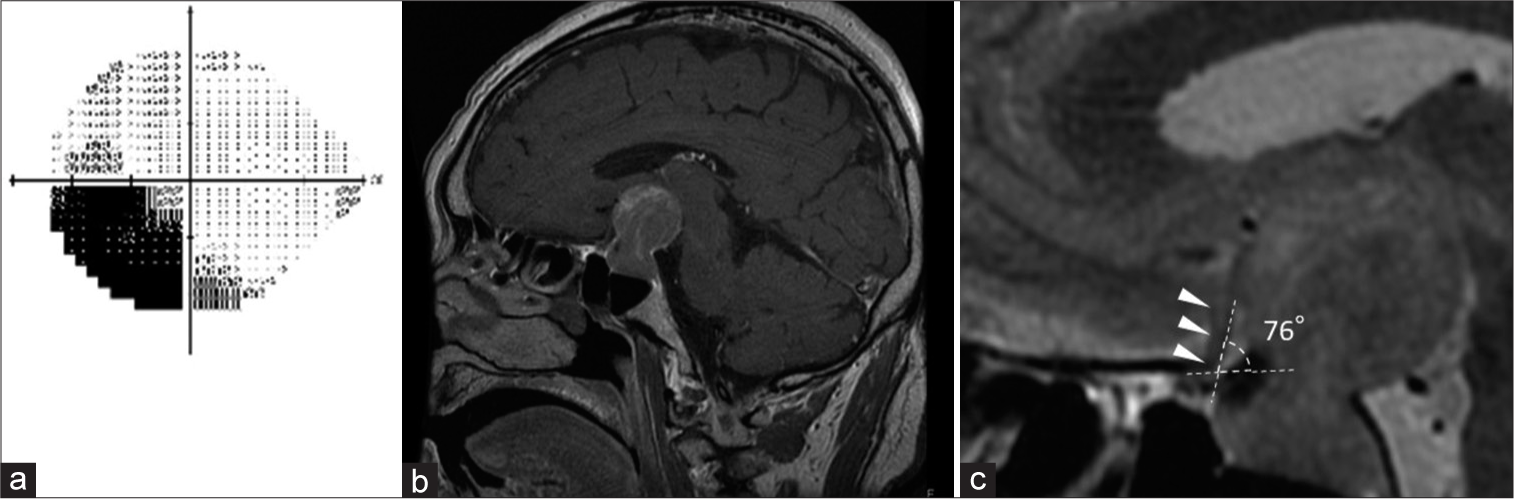
Figure 2:
The sagittal T2-weighted image demonstrates the sagittal optic nerve-canal bending angle (ONCBA) formed by the optic nerve in the optic canal and the intracranial subarachnoid space at the junction (arrowheads). The two dotted lines represent extensions of the optic nerve in the optic canal and subarachnoid space whose intersection determines the ONCBA.
Surgical techniques
All patients underwent transnasal transsphenoidal surgery using microscopic or endoscopic techniques. Subcapsular removal was performed. When possible, the tumors were dissected using the pseudocapsular technique. In cases of intraoperative cerebrospinal fluid leakage, small pieces of abdominal fat and/or fascia were packed into the tumor cavity. Lumbar cerebrospinal fluid drainage was performed after surgery at the operator’s discretion, according to the grading system reported by Esposito et al.[
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences software (IBM, Armonk, NY, USA). The Mann–Whitney U-test was used to compare continuous data (tumor height), and the Chi-square test was used to compare categorical data (Knosp grade) between two groups. Statistical significance was set at P < 0.05.
RESULTS
Patient and clinical characteristics
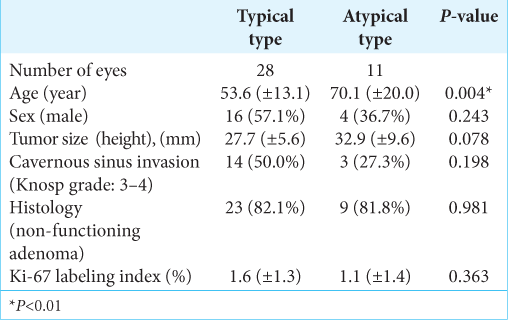
Data from 266 of the 440 evaluated eyes were excluded due to the absence of visual field defects. Out of 174 eyes with visual field defects, 135 (77.6%) were excluded because temporal hemianopia had already occurred. Temporal quadrantanopia was observed in 39 eyes (22.4%), of which 28 (16.1%) and 11 (6.3%) had typical and atypical defects, respectively. The clinical characteristics of the patients in these groups are shown in
The mean ages of the patients in the typical and atypical groups were 53.6 years (range 26–79 years) and 70.1 years (range 27– 90 years), respectively (P = 0.004). There was no significant difference in the male-to-female ratios between the two groups (P = 0.243). Tumor heights were also not significantly different between the two groups (27.7 ± 5.6 mm vs. 32.9 ± 9.6 mm, P = 0.078). Moreover, there were no statistically significant differences between the groups in terms of cavernous invasion (P = 0.198), histology (P = 0.981), and Ki-67 labeling index (1.6% ± 1.3% vs 1.1% ± 1.4%, P = 0.363).
Radiological evaluation
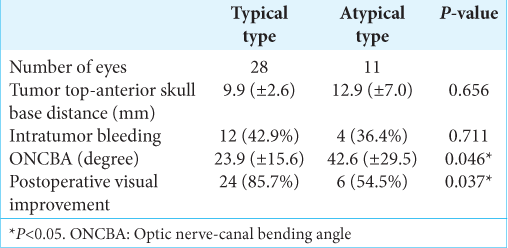
All the acquired images provided acceptable diagnostic image quality. A summary of the radiological evaluations of the tumor and its relationship with the surrounding tissues on MRI is shown
Tumor top-anterior skull base vertical distance (9.9 ± 2.6 mm vs. 12.9 ± 7.0 mm, P = 0.656) and intratumoral bleeding (P = 0.711) were not significantly related to the type of visual defects. ONCBA in the atypical group was significantly larger than that in the typical group (42.6° ± 29.5° vs. 23.9° ± 15.6°, P = 0.046) [
Figure 3:
An illustrative case of atypical visual field defects due to a bent optic nerve. (a) The preoperative Humphrey visual field test shows lower quadrantanopia in the left eye. (b) The sagittal gadolinium-enhanced T1-weighted image shows a pituitary adenoma with suprasellar extension. (c) The sagittal T2- weighted image demonstrates that the left optic nerve is bent (optic nerve-canal bending angle: 76°) due to the tumor (arrowheads). At the exit of the optic canal, the upper surface of the nerve appears to be compressed by the bony structure.
Visual outcome
The postoperative visual outcomes are reported [
DISCUSSION
We first analyzed the anatomical relationship around the optic nerve in cases of atypical visual field defects caused by pituitary adenoma. We found excessive bending of the optic nerve where it entered the optic tract to be common in patients with atypical visual field defects due to pituitary adenomas.
Visual field defects caused by pituitary tumors typically present as bilateral hemianopia, which is thought to arise superiorly and progress inferiorly.[
The underlying mechanism of pituitary adenoma-induced inferior dominant visual field defects may involve demyelination or ischemia. Excessive bending of the optic nerve, which is lifted upward by the tumor at the bony margin of the optic canal exit, results in a strong compression of the superior surface of the optic nerve. In animal studies, continuous compression of the optic nerve leads to demyelination. The remyelinated fibers observed after continuous nerve compression coexist with completely demyelinated fibers and do not appear to revert to their normal thickness and tissue structure.[
In our study, the superior surface of the optic nerve, which could be a finding supporting this study, could not be observed intraoperatively using transsphenoidal surgery; however, there have been reports of intraoperative visualization of the optic nerve during craniotomy for suprasellar meningiomas. Shapey et al. performed a craniotomy in a case of suprasellar meningioma with inferiorly dominated visual field defects and found a trace of compression on the superior surface of the optic nerve by the falciform ligament.[
Visual field defects caused by pituitary tumors can be improved by decompression of the optic nerve with surgical removal of the tumor.[
The study’s limitations are its retrospective design and inclusion of a small patient population. Another limitation is the lack of long-term follow-up. As mentioned above, it is possible that the atypical group with poor visual outcomes in this study may also have improved their visual field impairment in the long term. It is important to examine the long-term visual outcomes and compare them between the two groups, which can lead to different results from the current study. In addition, kinetic vision testing was only used in some patients. Although static vision testing is a well-established and commonly used quantitative method to evaluate visual field defects in clinical practice, future analyses using kinetic vision testing would strengthen our findings.
CONCLUSION
Excessive bending of the optic nerve at the edge of the optic canal observed using preoperative MRI is associated with inferior temporal quadrantanopia due to pituitary adenoma. Even if atypical visual field defects are found after visual field testing, pituitary tumors cannot be ruled out.
Ethical approval
The Institutional Review Board has approved the study protocol (No. 2014032) dated on June 19, 2019.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Altafulla JJ, Iwanaga J, Kikuta S, Prickett J, Ishak B, Uz A. The falciform ligament: Anatomical study with microsurgical implications. Clin Neurol Neurosurg. 2020. 195: 106049
2. Biousse V, Newman NJ. Ischemic optic neuropathies. N Engl J Med. 2015. 372: 2428-36
3. Clifford-Jones RE, Landon DN, McDonald WI. Remyelination during optic nerve compression. J Neurol Sci. 1980. 46: 239-43
4. Clifford-Jones RE, McDonald WI, Landon DN. Chronic optic nerve compression. An experimental study. Brain. 1985. 108: 241-62
5. Comtois R, Beauregard H, Somma M, Serri O, Aris-Jilwan N, Hardy J. The clinical and endocrine outcome to trans-sphenoidal microsurgery of nonsecreting pituitary adenomas. Cancer. 1991. 68: 860-6
6. Danesh-Meyer HV, Wong A, Papchenko T, Matheos K, Stylli S, Nichols A. Optical coherence tomography predicts visual outcome for pituitary tumors. J Clin Neurosci. 2015. 22: 1098-104
7. Dekkers OM, de Keizer RJ, Roelfsema F, Vd Klaauw AA, Honkoop PJ, van Dulken H. Progressive improvement of impaired visual acuity during the first year after transsphenoidal surgery for non-functioning pituitary macroadenoma. Pituitary. 2007. 10: 61-5
8. Dekkers OM, Pereira AM, Roelfsema F, Voormolen JH, Neelis KJ, Schroijen MA. Observation alone after transsphenoidal surgery for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab. 2006. 91: 1796-801
9. Esposito F, Dusick JR, Fatemi N, Kelly DF. Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery. Oper Neurosurg (Hagerstown). 2007. 60: 295-303 discussion 303-4
10. Gnanalingham KK, Bhattacharjee S, Pennington R, Ng J, Mendoza N. The time course of visual field recovery following transphenoidal surgery for pituitary adenomas: Predictive factors for a good outcome. J Neurol Neurosurg Psychiatry. 2005. 76: 415-9
11. Guler TM, Yilmazlar S, Ozgun G. Anatomical aspects of optic nerve decompression in transcranial and transsphenoidal approach. J Craniomaxillofac Surg. 2019. 47: 561-9
12. Hayreh SS. Ischemic optic neuropathy. Prog Retin Eye Res. 2009. 28: 34-62
13. Hollenhorst R, Younge B, Kohler PO, Ross GT, editors. Ocular manifestations produced by adenomas of the pituitary gland analysis of 1000. New York: Elsevier; 1973. p.
14. Jakobsson KE, Petruson B, Lindblom B. Dynamics of visual improvement following chiasmal decompression. Quantitative pre-and postoperative observations. Acta Ophthalmol Scand. 2002. 80: 512-6
15. Kerrison JB, Lynn MJ, Baer CA, Newman SA, Biousse V, Newman NJ. Stages of improvement in visual fields after pituitary tumor resection. Am J Ophthalmol. 2000. 130: 813-20
16. Kim JH, Kim CY, Yang HK, Hwang JM. Unusual chiasmal visual field defects. Neurol Sci. 2013. 34: 2057-60
17. Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: A magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993. 33: 610-7 discussion 617-8
18. Lee IH, Miller NR, Zan E, Tavares F, Blitz AM, Sung H. Visual defects in patients with pituitary adenomas: The myth of bitemporal hemianopsia. AJR Am J Roentgenol. 2015. 205: W512-8
19. Ogra S, Nichols AD, Stylli S, Kaye AH, Savino PJ, Danesh-Meyer HV. Visual acuity and pattern of visual field loss at presentation in pituitary adenoma. J Clin Neurosci. 2014. 21: 735-40
20. Schiefer U, Isbert M, Mikolaschek E, Mildenberger I, Krapp E, Schiller J. Distribution of scotoma pattern related to chiasmal lesions with special reference to anterior junction syndrome. Graefes Arch Clin Exp Ophthalmol. 2004. 242: 468-77
21. Shapey J, Danesh-Meyer HV, Kaye AH. Suprasellar meningioma presenting with an altitudinal field defect. J Clin Neurosci. 2012. 19: 155-8
22. Smith KJ, Blakemore WF, McDonald WI. The restoration of conduction by central remyelination. Brain. 1981. 104: 383-404
23. Traustason OI, Feldon SE, Leemaster JE, Weiner JM. Anterior ischemic optic neuropathy: Classification of field defects by Octopus automated static perimetry. Graefes Arch Clin Exp Ophthalmol. 1988. 226: 206-12
24. Wang H, Sun W, Fu Z, Si Z, Zhu Y, Zhai G. The pattern of visual impairment in patients with pituitary adenoma. J Int Med Res. 2008. 36: 1064-9
25. Wang MT, King J, Symons RC, Stylli SS, Meyer J, Daniell MD. Prognostic utility of optical coherence tomography for long-term visual recovery following pituitary tumor surgery. Am J Ophthalmol. 2020. 218: 247-54
26. Yamaguchi R, Tosaka M, Miyagishima T, Osawa T, Horiguchi K, Honda F. Sagittal bending of the optic nerve at the entrance from the intracranial to the optic canal and ipsilateral visual acuity in patients with sellar and suprasellar lesions. J Neurosurg. 2019. 134: 180-8