- Department of Neurosurgery, Manchester Centre for Clinical Neurosciences, Salford, United Kingdom
- Department of Biology, Medicine and Health, University of Manchester, Manchester, United Kingdom
Correspondence Address:
Francesca Colombo, Department of Neurosurgery, Manchester Centre for Clinical Neurosciences, Salford, United Kingdom.
DOI:10.25259/SNI_126_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Francesca Colombo1, Ross McLeod1, Rohit Ravindranath Nambiar1, Helen Maye1, Sam Dickens1, K. Joshi George1,2. Informed consent in neurosurgery – Evaluation of current practice and implementation of future strategies. 19-Jul-2024;15:246
How to cite this URL: Francesca Colombo1, Ross McLeod1, Rohit Ravindranath Nambiar1, Helen Maye1, Sam Dickens1, K. Joshi George1,2. Informed consent in neurosurgery – Evaluation of current practice and implementation of future strategies. 19-Jul-2024;15:246. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13000
Abstract
Background: In recent times, clinical negligence claims against National Health Service hospitals have doubled, with 8% of claims being made due to “failure to warn/informed consent.” This study aimed to assess the current compliance of the neurosurgical division within a large tertiary neuroscience center with the national legal framework and professional guidelines around the issue of surgical consent and to develop strategies to improve the consent process.
Methods: Electronic patient records (EPR) were accessed to collect demographic data and information regarding the surgical procedures. Telephone questionnaires were carried out. Neurosurgical registrars were interviewed. The author met with the trust’s Legal team, the neuropsychology lead, and the trust’s consent lead.
Results: Fifty-eight patients were included in the analysis. Of the respondents to the questionnaire, 98% felt that they were adequately informed during the consent process. When consenting patients, all registrars felt that they explained the reason for the procedure, detailed benefits, and major risks, including uncommon and rare risks. However, 50% admitted to not specifically discussing the postoperative recovery time or alternatives. Only 15% admitted to documenting on the EPR or through a letter to the patient’s General Practitioner.
Conclusion: Informed consent is a delicate moment of communication between a clinician and the patient. Regular training and good communication skills help staff to focus on the most relevant aspects of consent, which should be delivered in an appropriate environment and with family support. Audio-visual aids can support the process but do not replace good communication.
Keywords: Informed consent, Litigation, Neurosurgery, Test of materiality, Three-legged stool
INTRODUCTION
Neurosurgery is one of the highest risk specialties for malpractice within the National Health Service (NHS), second only to Obstetrics and Gynecology.[
Consent is central to everything that occurs in healthcare, be that the consent needed to examine a patient or consent for a procedure. The principle of consent reflects the patient’s right to determine what happens to their body both when having physical examinations and when undergoing investigations and procedures.[
In recent times, there has been a doubling of clinical negligence claims against NHS hospitals,[
Informed consent claims are usually brought in the tort of negligence; not negligence in carrying out the treatment but negligence in advising the patient about the risks. Two fundamental elements are needed in order for the claim to be successful: [
Before obtaining consent, the surgeon must provide the patient with information about alternative treatments and the risks of not undergoing surgery. The surgeon must then explain the nature of the proposed surgery, the intended benefits, material risks (to that particular patient), and potential complications. Surgeons must use their clinical judgment in discussion with the patient[
While the responsibility for ensuring a valid consent process has taken place remains with the doctor, the process of consent now requires the more active involvement of the patient, taking into account not just their profession but also their hobbies, interests, and general wishes when recommending a course of treatment.[
Other studies around the matter of consent have been conducted internationally, such as the ones by Anderson and Wearne,[
NHS organizations must provide a framework to ensure that Trusts comply with regulatory requirements, that a standard of care is established, and that employees are supported in their work. The lack of standardized guidelines represents a risk to individual health-care professionals and NHS Trusts. Failing to obtain informed consent may result in disciplinary action from the employing Trust and/or professional bodies[
This study aimed to assess the current compliance of the neurosurgical division within a large tertiary neuroscience center with the national legal framework and professional guidelines around the issue of surgical consent and to develop divisional wide clinician and patient-centered strategies to improve the consent process. The results of this study are generalizable to other centers and other surgical specialties.
MATERIALS AND METHODS
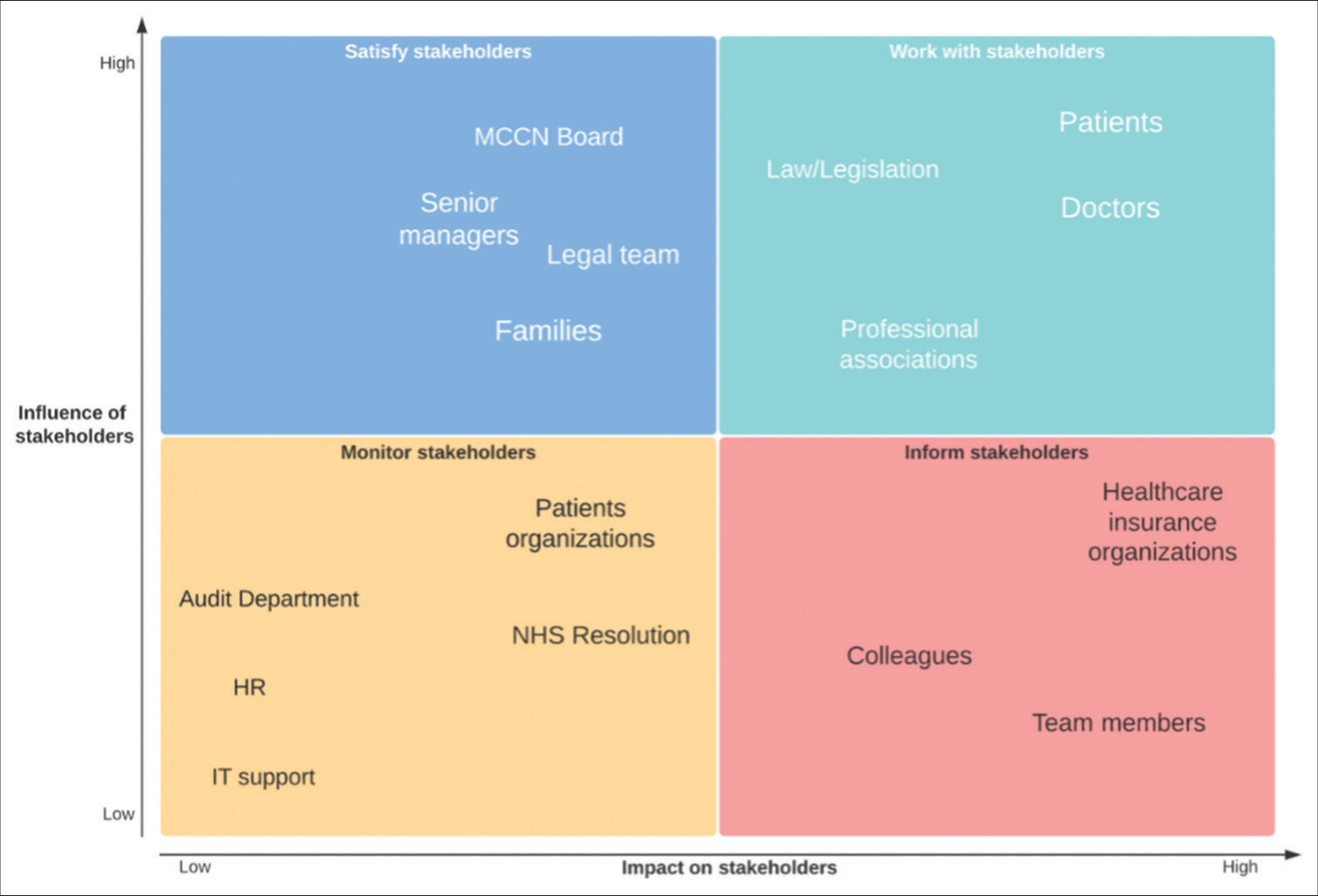
During the preliminary phases of the study, a stakeholder analysis was carried out to ascertain the key components of the consent process and inform the rest of the project [
The project was registered with the trust’s audit and legal departments. Telephone consent was obtained from all patients taking part in the telephone questionnaire. Electronic patient records (EPR) were accessed to collect demographic data and information regarding the surgical procedure. Telephone questionnaires were carried out to ascertain the patient’s understanding of the procedure and whether the patient fully understood the risks and benefits of surgery, including alternative treatments and the emotional and physical impact of the complication. Patients were also asked about their thoughts on how to improve the consent process. Patients who underwent emergency surgery were excluded as both the timing and context of the consent process differ significantly between elective surgery and emergency/life threatening surgery.[
The questionnaire was designed de novo by the researcher, with question choice being informed by a literature review around the matter of consent, in particular from studies that reference optimal consent practices.[
Questions 1–6 refer to the overall satisfaction with the consent process and the context of where the consent process took place. Question 7 refers to the test of materiality. Questions 8–14 refer to the “three-legged stool.” Questions 15–19 are related to the patient’s perception of the consent process and their opinion on how we could improve this.
Neurosurgical registrars were interviewed [
The author met with the trust’s Legal team to discuss issues pertaining to consent and obtained data from NHS resolution on previous (within the past 5 years) and ongoing litigation in the trust. The author also met with the neuropsychology lead to ascertain the impact of consent on the patient and to discuss factors that can increase a patient’s understanding of the consent process and how this can be enhanced. Discussions were held with the trust’s consent to gain an understanding of current trust policies and the results of previous consent audits.
RESULTS
The patients
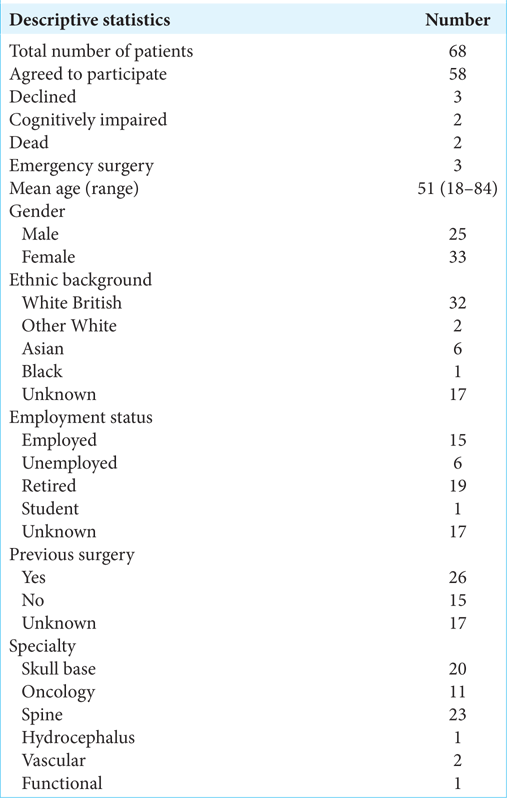
Sixty-eight patients were identified to have suffered a complication during the study period and were eligible to participate in this study. Three declined to participate, two were cognitively impaired at the time of consent, and therefore, surgery was carried out with a consent form 4, three had undergone emergency surgery, and two had subsequently died, leaving 58 patients in the analysis group. About 71% of this group filled in the telephone questionnaire. This study is focused on qualitative descriptors with the aim of understanding the patient’s experience of the consent process; therefore, we do not believe that the analysis of the patient’s response would be invalidated by the lack of response of the 17 patients who did not fill in the questionnaire. The results that have been presented are based on the answers of the patients who did respond to the questionnaire.
The mean age of the patient was 51 years (range 18–84). About 57% of participants were female, while 55% were from a White British ethnic background. About 26% were employed, while 33% were retired at the time of surgery [
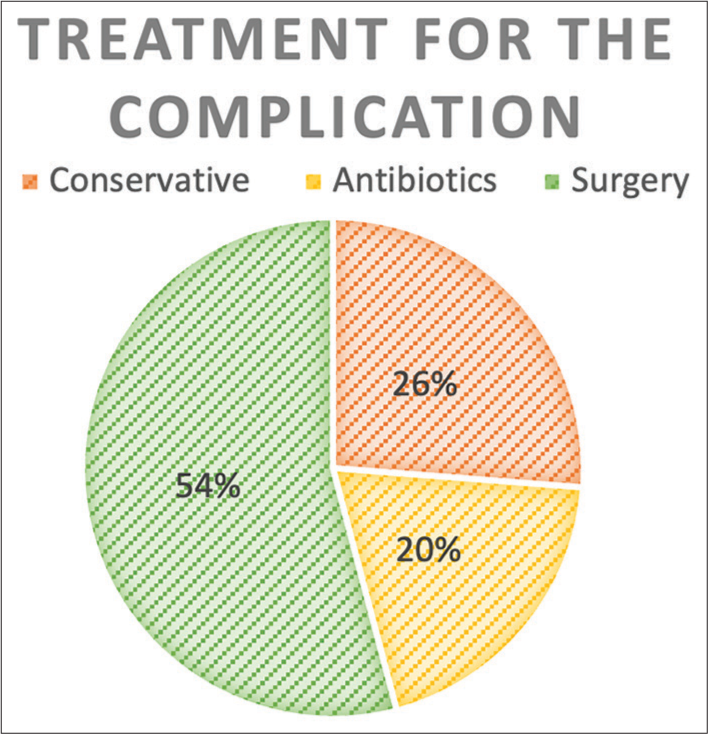
Of the respondents to the questionnaire [
About 17% of patients felt that not enough emphasis was put on postoperative care and recovery time. All patients felt that they understood the indications and benefits of surgery, and only one patient felt that they did not understand the major risks. About 68% of patients felt that they understood what a complication would mean to them and what this may mean in terms of ongoing care.
About 83% of our patients received either an information leaflet or a QR code from the clinic, although only 59% received a copy of their consent form in the clinic.
All patients had consent confirmed on the morning of surgery, with only one patient feeling that they were not given sufficient opportunity to ask questions. Despite having had a complication, 90% of patients, when questioned, would choose to have the operation again.
The healthcare professionals
Eighteen of the 19 neurosurgery registrars responded. Their average surgical experience was 6 years (range 3–14 years). Only 22% had had formal consent training. About 50% were aware of the Bolam test, 56% of the materiality test, 72% of the ethical principles, and 78% of the General Medical Council (GMC) guidance. Nobody had read or knew about the trust consent policy. When consenting patients, all registrars felt that they explained the reason for the procedure, detailed benefits, and major risks, including uncommon and rare risks. However, 50% admitted to not specifically discussing the postoperative recovery time or alternatives.
About 39% stated that they always gave a copy of the consent form to take home; only 17% always gave information leaflets, with many being unaware that these resources existed. Only 15% admitted to documenting on the EPR or through a letter to the patient’s General Practitioner. However, the registrars were generally only reconfirming consent rather than starting the process of consent. The consultants were the ones starting the consent process in the clinic and they had documentation about what was discussed and sent to the patient. All clinicians felt that they offered patients opportunities to ask questions about the procedure or its complications and felt that they discussed consent in lay terms with a good rapport with the patient. Common themes to improve the consent process include information leaflets or online resources, the training of doctors, consent clinics either through phone or in person, access to specialist nurses at all stages, and more time to carry out the consent consultation.
All patients, apart from four, were verbally consented to the clinic by the responsible consultant. The risks and benefits of the procedure were discussed and documented in the clinical letters. The four patients who did not have a clinical letter documenting the consent process were oncology patients who were admitted to the ward directly following a multidisciplinary meeting discussion.
NHS resolution
Three claims were made against the trust between January 2016 and December 2021. In all the cases, failure to consent was not the reason for the claim or the pay-out but just collateral to the main complaint.
The audit department
A trust-wide consent audit is undertaken annually by all specialties, which is then reported at a divisional level. The overall performance of the department has been satisfactory. Areas for improvement include documentation of demographics, evidence either on EPR or on the consent form that information leaflets have been provided, and evidence that the clinician has checked the patient’s understanding. Most times the patient did not receive a copy of the consent.
A strengths, weaknesses, opportunities, and threats analysis was carried out utilizing the results of the patient/registrar questionnaires, trust audit department, and NHS litigation [
Neuropsychology input
The consultant lead for neuropsychology was interviewed through video-consultation. Her suggestions included:
Where possible, a relative of the patient should be present during the consultation, to absorb some of the emotional distress and provide reassurance to the patient. The relative can also help to retain information and relay it to the patient at a later stage. Ensure leaflets/take home materials are available and include risks, alternative treatments, and an explanation of the most relevant complications. Clinicians responsible for the consent process should undertake legal/ethical mandatory training and communication skills training. Specialist nurses play a fundamental role in supporting patients in the pre-and post-operative period.
DISCUSSION
The need for informed consent arises from the legal concept of battery and the ethical principle of autonomy. In 1985, the Bolam test was questioned publicly for the 1st time after the ruling of Sidaway versus Bethlem Royal Hospital Governors.[
This paternalistic culture was further called into question in 2004 when Chester versus Afshar was found in favor of the patient[
This case recognized the shift in health care from a paternalistic culture to a partnership between the patient and the doctor, with the patient having the right to know all risks that would be relevant to them as an individual. This test of materiality has replaced what the reasonable doctor would do with what the reasonable patient would want to know.[
The main ethical consideration related to informed consent is the concept of autonomy. Patients have the right to determine what treatment they consent to, and doctors must respect their wishes.[
This study demonstrated that the consent process within the neurosurgery department of our trust is overall satisfactory, though there were areas for improvement. This is evidenced by the overall satisfaction of patients with the consent process, as demonstrated by the patient questionnaires and very small numbers of litigation cases related to failure to consent.
Our trust has already implemented several strategies to support the consent process, such as an up-to-date consent policy available online, leaflets, and QR codes for some of the most common procedures. Yearly consent audits are carried out and consent is also reconfirmed on the morning of surgery to offer a further opportunity for patients to clarify anything that they are unsure of. The literature reveals a multitude of issues that can cause the consent process to fail, including too much or not enough information, too much medical jargon, information leaflets pitched at a level the patient cannot understand, and time pressures, to name but a few.[
COVID-19 had a significant impact on standard practice throughout the UK, as a limited number of face-to-face clinics were available, and family members were not allowed to come into the hospital. The patients’ questionnaire and neuropsychology input have highlighted the importance of family support and face to face consultations for an optimal consent process.
The report from the NHS resolution and the findings of the clinicians’ questionnaire have been discussed with the Legal team that supports the trust. Consent training has been identified as an area of weakness. More importance needs to be given to the patient’s understanding of the complications of a procedure and how these may affect them as an individual/their quality of life. Registrars need to be made aware that, further to signing the consent form, they need to record the encounter with either a clinic letter or a note on EPR.
The findings of this study have once again confirmed the invaluable contribution of specialist nurses. Patients value the possibility of reaching out to them pre-and postoperatively, as they play an irreplaceable role in reassuring patients and their families and answering questions/conveying information. The lack of patient support groups has been highlighted and attempts are being made to try and re-establish these in some specialties in the post-COVID era.
As a result of the project, areas of improvement were identified that could be applied to most hospital trusts to improve the consent process. These include:
Regular consent teaching sessions for clinicians, supported by the trust legal team. Topics should be based around the “three-legged stool” and should include the legal and ethical principles of consent and the importance of accurate documentation These sessions could be recorded and included as part of mandatory training on Induction of new registrars Clinicians should be encouraged to attend communication skills courses Face to face consultations should be the preferred modality when patients need to be consented. Family members should be encouraged to attend “Consent clinics” have been considered as a possible strategy, but they pose issues in terms of staffing and the availability of clinical space. Allied healthcare professionals such as physiotherapists or neuropsychologists may be able to offer support to either deliver information or confirm consent. Leaflets and QR codes can be developed to cover more procedures Patients who had experienced prolonged hospital stays and complications could lead patient support groups More specialist nurses in different subspecialties can offer further support to patients and families Electronic consent pathways will be helpful in reducing paperwork, facilitating recording of the process, and helping shared decision making.
CONCLUSION
Informed consent is a delicate moment of communication between a clinician and the patient. Regular training and good communication skills help staff to focus on the most relevant aspects of consent, which should be delivered in an appropriate environment and with family support. Audio-visual aids can support the process but do not replace good communication.
Ethical approval
The research project has been registered with the Hospital’s Audit department and has been conducted according to the ethical principles of the Declaration of Helsinki for medical research (The World Medical Association 2008).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Akkad A, Jackson C, Kenyon S, Dixon-Woods M, Taub N, Habiba M. Informed consent for elective and emergency surgery: Questionnaire study. BJOG. 2004. 111: 1133-8
2. Anderson OA, Wearne IM. Informed consent for elective surgery: What is best practice?. J R Soc Med. 2007. 100: 304-5
3. Coulter AA, Collins A, editors. Making shared decision-making a reality. London: The Kings Fund; 2011. p. 1-56
4. Department of Heal, editors. Reference guide to consent for examination or treatment. 2012. p.
5. General Medical, editors. Decision making and consent. Guidance on professional standards and ethics for doctors. 2020. p. 13-20
6. Glaser J, Nouri S, Fernandez A, Sudore RL, Schillinger D, Klein-Fedyshin M. Interventions to improve patient comprehension in informed consent for medical and surgical procedures: An updated systematic review. Med Decis Making. 2020. 40: 119-43
7. Gov., editors. Health and Social Care Act. 2012. p.
8. Hamdan A, Strachan RD, Nath F, Coulter IC. Counting the cost of negligence in neurosurgery: Lessons to be learned from 10 years of claims in the NHS. Br J Neurosurg. 2015. 29: 169-77
9. Hanson M, Pitt D. Informed consent for surgery: Risk discussion and documentation. Can J Surg. 2017. 60: 69-70
10. Lord Templeman, Lord Diplock, Lord Scaraman, Keith L, editors. Sidaway v Board of governors of the bethlmen royal hospital and the maudsley hospital. 1985. p.
11. Machin JT, Hardman J, Harrison W, Briggs TW, Hutton M. Can spinal surgery in England be saved from litigation: A review of 978 clinical negligence claims against the NHS. Eur Spine J. 2018. 27: 2693-9
12. McCormack DJ, Gulati A, Mangwani J. Informed consent: A global perspective. Bone Joint J. 2018. 100B: 687-92
13. McKinnon C, Loughran D, Finn R, Coxwell-Matthewman M, Jeyaretna DS, Williams AP. Surgical consent practice in the UK following the Montgomery ruling: A national cross-sectional questionnaire study. Int J Surg. 2018. 55: 66-72
14. M, editors. New guidance on decision making and consent. 2020. p.
15. Ministry of Ethi, editors. Consent and confidentiality. 2014. p.
16. Parliament , editors. Judgments - chester v. afshar. 2004. p.
17. Perrenoud B, Velonaki VS, Bodenmann P, Ramelet AS. The effectiveness of health literacy interventions on the informed consent process of health care users: A systematic review protocol. JBI Databse Sys Rev Implement Rep. 2015. 13: 82-94
18. Selinger CP. The right to consent: Is it absolute?. Br J Med Pract. 2009. 2: 50-4
19. Steele L, Mukherjee S, Stratton-Powell A, Anderson I, Timothy J. Extent of medicolegal burden in neurosurgery-an analysis of the National Health Service Litigation Authority Database. Br J Neurosurg. 2015. 29: 622-9
20. Wald D, editors. Sharp rise in NHS negligence claims for lack of informed consent. 2020. p.
21. Wald DS, Casey-Gillman O, Comer K, Mansell JS, Teoh ZH, Mouyis K. Animation-supported consent for urgent angiography and angioplasty: A service improvement initiative. Heart. 2020. 106: 1747-51
22. Weckbach S, Kocak T, Reichel H, Lattig F. A survey on patients’ knowledge and expectations during informed consent for spinal surgery: Can we improve the shared decision-making process?. Patient Saf Surg. 2016. 10: 10-3
23. Wheeler R. Consent in surgery. Ann R Coll Surg Engl. 2006. 88: 261-4