- Department of Neurosurgery, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
- School of Medicine, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
- Faculty of Medicine, Ain Shams University, Cairo, Egypt.
- Department of Neurosurgery, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
Correspondence Address:
Ahmed Kamel Mohamed Moner Basha, Department of Neurosurgery, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
DOI:10.25259/SNI_609_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Seif Tarek El-Swaify1, Menna Kamel2, Sara Hassan Ali2, Bassem Bahaa3, Mazen Ahmed Refaat3, Abdelrahman Amir2, Abdelrahman Abdelrazek2, Pavly Wagih Beshay2, Ahmed Kamel Mohamed Moner Basha4. Initial neurocritical care of severe traumatic brain injury: New paradigms and old challenges. 23-Sep-2022;13:431
How to cite this URL: Seif Tarek El-Swaify1, Menna Kamel2, Sara Hassan Ali2, Bassem Bahaa3, Mazen Ahmed Refaat3, Abdelrahman Amir2, Abdelrahman Abdelrazek2, Pavly Wagih Beshay2, Ahmed Kamel Mohamed Moner Basha4. Initial neurocritical care of severe traumatic brain injury: New paradigms and old challenges. 23-Sep-2022;13:431. Available from: https://surgicalneurologyint.com/surgicalint-articles/11887/
Abstract
Background: Early neurocritical care aims to ameliorate secondary traumatic brain injury (TBI) and improve neural salvage. Increased engagement of neurosurgeons in neurocritical care is warranted as daily briefings between the intensivist and the neurosurgeon are considered a quality indicator for TBI care. Hence, neurosurgeons should be aware of the latest evidence in the neurocritical care of severe TBI (sTBI).
Methods: We conducted a narrative literature review of bibliographic databases (PubMed and Scopus) to examine recent research of sTBI.
Results: This review has several take-away messages. The concept of critical neuroworsening and its possible causes is discussed. Static thresholds of intracranial pressure (ICP) and cerebral perfusion pressure may not be optimal for all patients. The use of dynamic cerebrovascular reactivity indices such as the pressure reactivity index can facilitate individualized treatment decisions. The use of ICP monitoring to tailor treatment of intracranial hypertension (IHT) is not routinely feasible. Different guidelines have been formulated for different scenarios. Accordingly, we propose an integrated algorithm for ICP management in sTBI patients in different resource settings. Although hyperosmolar therapy and decompressive craniectomy are standard treatments for IHT, there is a lack high-quality evidence on how to use them. A discussion of the advantages and disadvantages of invasive ICP monitoring is included in the study. Addition of beta-blocker, anti-seizure, and anticoagulant medications to standardized management protocols (SMPs) should be considered with careful patient selection.
Conclusion: Despite consolidated research efforts in the refinement of SMPs, there are still many unanswered questions and novel research opportunities for sTBI care.
Keywords: Intracranial hypertension, Intracranial pressure monitoring, Neurocritical care, Neurotrauma, Thromboembolism prophylaxis, Traumatic brain injury
INTRODUCTION
Every 21 seconds, a traumatic brain injury (TBI) occurs in the USA.[
With the majority of sTBI patients being admitted to trauma, general, and surgical intensive care units (ICUs), standardized management protocols (SMPs) for sTBI may improve clinical outcomes regardless of where patients are admitted.[
Neurotrauma is a rapidly evolving field. Knowledge of the latest evidence may not directly change treatment decisions, but it will definitely guide future research initiatives. We are writing this review to highlight some recent advances and ongoing challenges in the early neurocritical care of sTBI since the publication of the 2017 Brain Trauma Foundation’s (BTF) guidelines.[
IDENTIFYING NEUROWORSENING
Any sTBI patient should be considered a critical patient with the potential risk of further deterioration beyond the initial insult, especially within the first 48 h. Critical neuroworsening is defined as a deterioration in the neurological status of the neurologically debilitated patient necessitating early recognition along with prompt evaluation and management.[
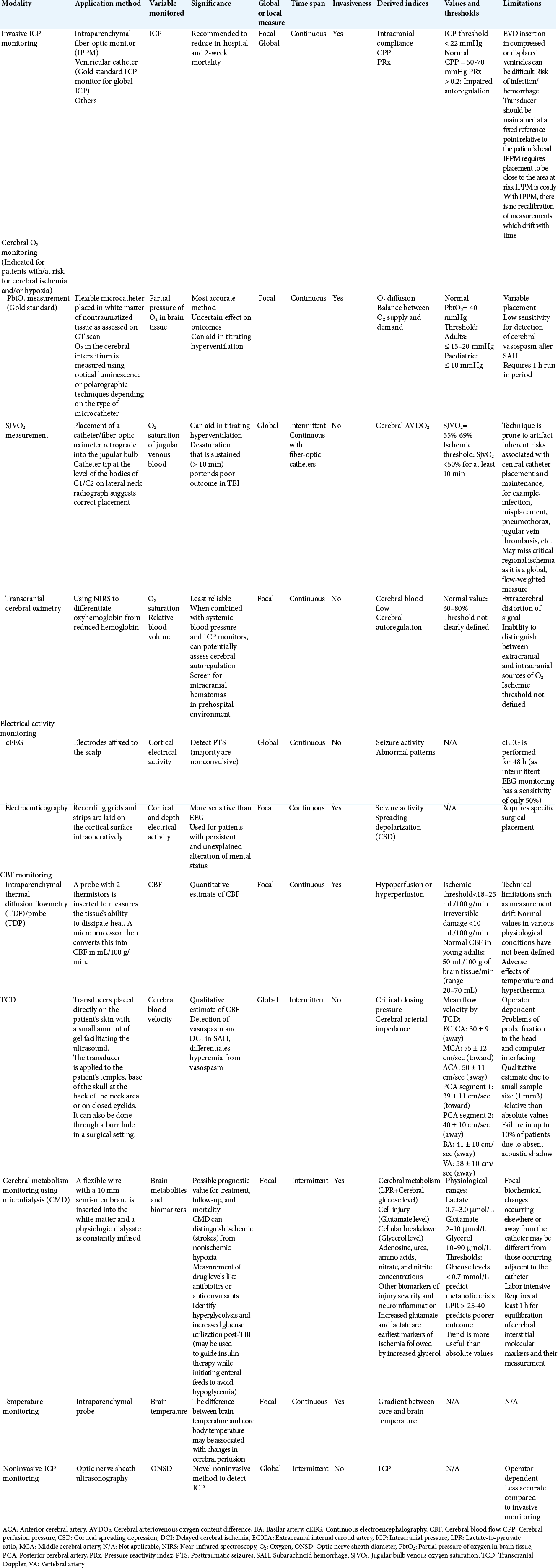
Multimodality neuromonitoring [
DEFINING THRESHOLDS
Most TBI patients are susceptible to altered cerebral autoregulation and detrimental increases in ICP with resultant fluctuations in cerebral blood flow (CBF).[
The variability in defining optimum thresholds has ignited interest in pursuing patient-specific measurements based on the physiology of cerebral vascular reactivity instead of targeting static ICP and CPP thresholds.[
MANAGING INTRACRANIAL HYPERTENSION (IHT)
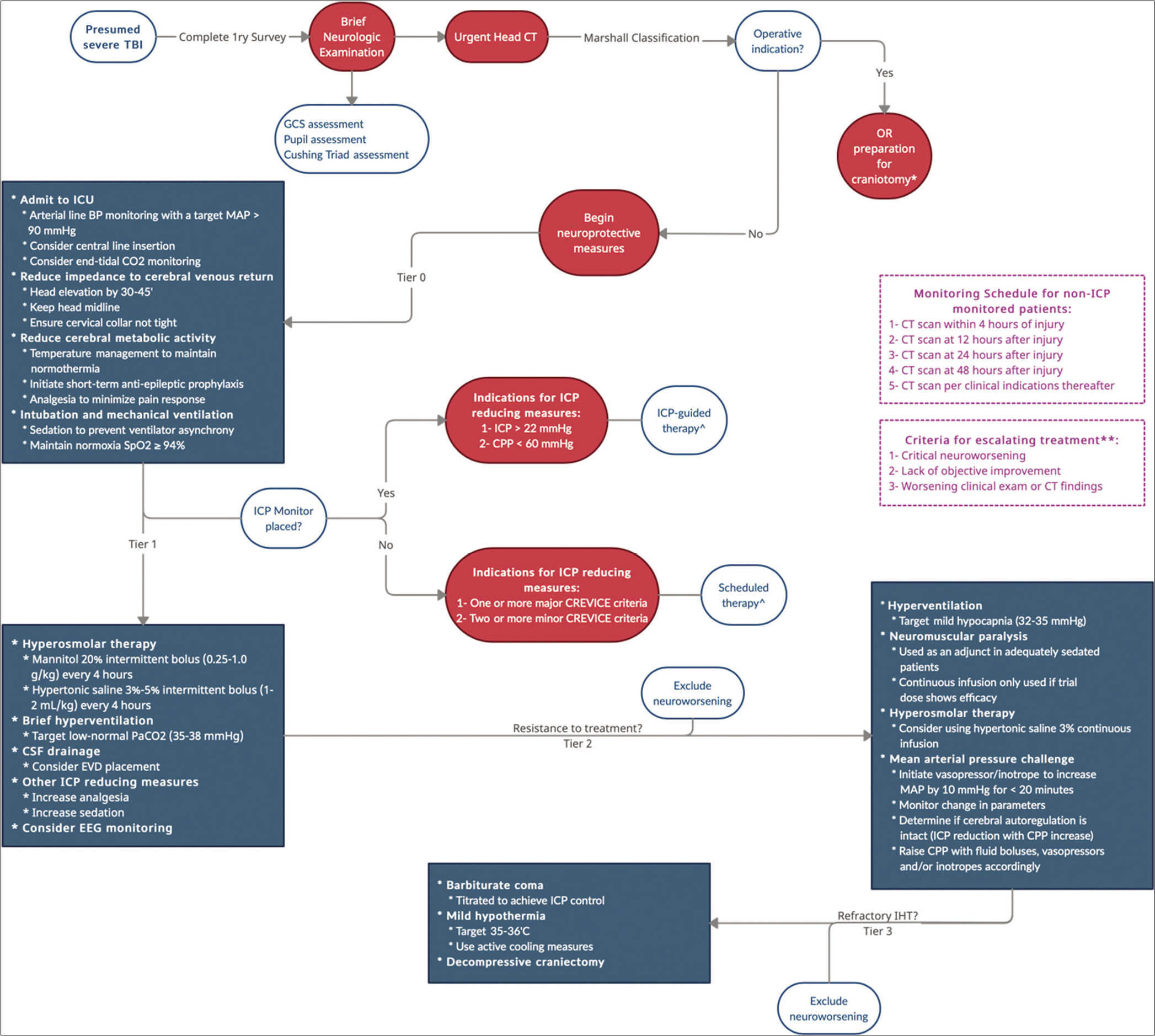
The initiation of ICP reducing measures is triggered when patients meet certain criteria and exceed prespecified thresholds. Maintaining these thresholds is a challenging task complicated by the lack of sufficient high-quality evidence as previously mentioned. The SIBICC adopted a consensus-based tier system to categorize the interventions commonly employed to prevent and control secondary IHT in patients with ICP monitors. Treatments within the same tier are employed without a specific order and are based on individual cases. Tier-zero, ideally initiated in the ICU, represents the primary management of patients to stabilize the condition and achieve neuroprotection regardless of the eventual ICP reading. Beyond tier-zero, the tiers target lowering the ICP according to the recommended thresholds by the BTF guidelines.[
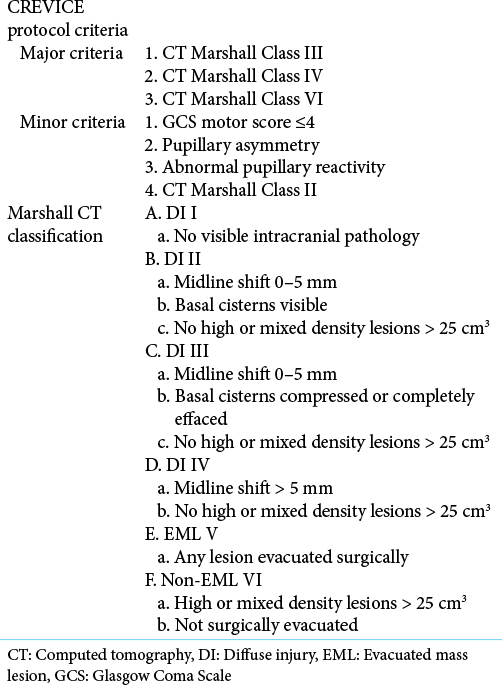
For patients who do not undergo ICP monitoring, especially in under-resourced LMICs, the Consensus Revised Imaging and Clinical Examination (CREVICE) Protocol employs a similar tier-based algorithm that is initiated based on major and minor criteria [
Figure 1:
Algorithmic approach to the management of raised intracranial pressure. BP: Blood pressure, CPP: Cerebral perfusion pressure, CT: Computed tomography, EEG: Electroencephalogram, EVD: External ventricular drain, GCS: Glasgow Coma Scale, ICP: Intracranial pressure, MAP: Mean arterial pressure, OR: Operating room. *A postoperative CT scan is obtained, and patients are reclassified using the Marshall classification using a dual system. **Escalating treatment is defined as using another measure from the same tier of therapy or upgrading to a higher tier. ^Refers to the dosing frequency of hyperosmolar therapy.
Hyperosmolar therapy still has many controversies with no recommendations regarding the type of agent (hypertonic saline [HTS] vs. mannitol) or concentrations to be used.[
A small subset of sTBI patients with IHT develops refractory IHT that is detrimental to cerebral physiology and disrupts all homeostatic mechanisms.[
Despite the differences in the thresholds employed for the intervention, both studies showed comparable results. Secondary DC performed significantly better in the reduction of ICP, reduction in use of ICP-lowering therapies, and reduction of ICU length of stay (LOS). Only the RESCUEicp study showed significantly lower 12-month mortality in the DC arm (30.4% vs. 52.0%). However, patients who survived had an increased incidence of poor functional outcomes in the DC group compared to the medical therapy group (DECRA = odds ratio [OR], 0.33; 95% CI 0.12–0.91). In light of these findings, the current recommendations are to perform secondary DC for late refractory ICP elevation to improve mortality and functional outcomes (level IIA) but not for early refractory ICP elevation.[
SHOULD ICP BE INVASIVELY MONITORED?
Elevated ICP is detrimental to cerebral physiology through reduction of CBF and compression or herniation of cerebral structures. Invasive cerebral monitoring in general and ICP monitoring in specific allow real-time assessment of cerebral physiology and dynamic titration of treatment modalities to optimize cerebral tissue conditions. Although ICP monitoring should logically be used for critical patients when available, the previous indications for ICP monitoring are no longer supported by the 2017 BTF guidelines, especially since prior guideline compliance was poor, and neither survival nor functional outcomes were significantly improved as evidenced in the landmark BEST: TRIP RCT (Benchmark Evidence from South American Trials: Treatment of ICP).[
Several recent large cohort studies have shown significantly lower mortality rates with ICP monitoring.[
PLACING THE ICP MONITOR
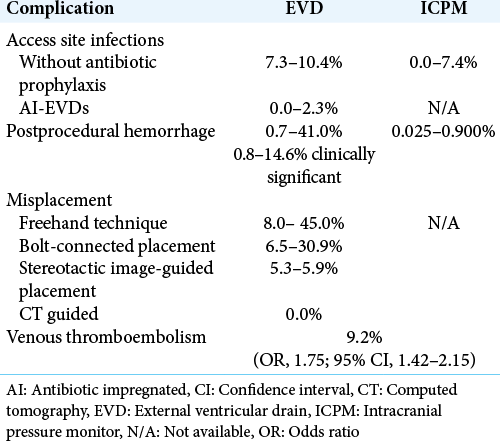
In the event that the neurocritical care team elects to place an ICP monitor, they will still be faced with several decisional dilemmas. The optimal ICP monitoring modality is still unknown. Historically, the superiority of the external ventricular drain (EVD) as an ICP monitoring modality was related to its accuracy, ability to recalibrate, cost-effectiveness, and the fact that it could be used as a therapeutic modality to lower ICP through cerebrospinal fluid drainage.[
The timing of ICP monitor placement is a matter of debate. Early ICP monitor placement, defined as within 6 h of admission, was not found to be associated with better mortality rates in neither adult nor pediatric patients.[
ANTI-SEIZURE PROPHYLAXIS AND NEUROPROTECTION
Posttraumatic seizures (PTSs) can potentially worsen the secondary brain injury by increasing cerebral metabolism and inducing neural excitotoxicity. They are subdivided into early PTS and late PTS. The incidence reaches 16.9% and 30.0%, respectively.[
Paroxysmal sympathetic hyperactivity (PSH), an important differential of PTS, is an underdiagnosed syndrome of adrenergic surge with characteristic symptoms of tachycardia, tachypnea, hypertension, hyperthermia, sweating, and posturing during paroxysmal episodes. Up to 80% of cases of PSH are due to TBI and around 10% of patients with TBI suffer from PSH. PSH portends an unfavorable prognosis up to death due to long ICU stays, longer mechanical ventilation time, increased infectious episodes, and worse GOS scores.[
A consolidated research effort is being put into the development of effective neuroprotective agents that can improve long-term outcomes after TBI. The discussion of the potential neuroprotective role of beta-blockers merits a short discussion of another promising neuroprotective agent: amantadine. Amantadine is an indirect dopamine agonist and N-methyl-D-aspartate antagonist. It is believed to improve posttraumatic cognitive function and accelerate functional recovery by replenishing depleted dopamine in neural circuits responsible for attentional and arousal functions (frontostriatal, nigrostriatal, and mesolimbic circuits). A recent meta-analysis of 20 studies found that amantadine significantly improved cognitive function (standardized MD (SMD), 0.50; 95% CI, 0.33-0.66), especially if administered in the 1st week (SMD, 0.97; 95% CI, 0.45–1.49) for <1 month (SMD, 0.83; 95% CI, 0.56–1.11). The effect in sTBI specifically was still statistically significant (SMD, 0.45; 95% CI, 0.11–0.78).[
THROMBOEMBOLISM PROPHYLAXIS
In general, trauma is a hypercoagulable state, and TBI specifically leads to a systemic coagulopathy due to the release of procoagulant molecules and platelet-activating molecules in addition to prolonged immobilization.[
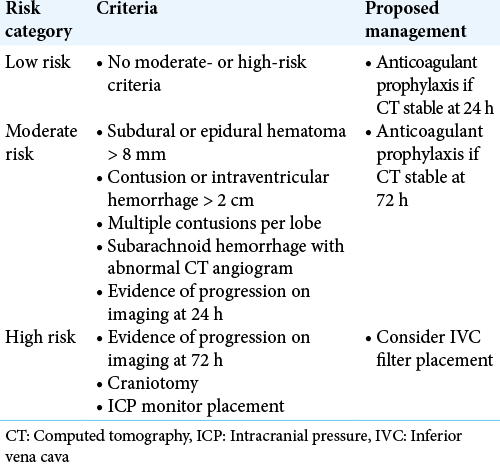
Several systematic reviews have highlighted the safety of early initiation of anticoagulants provided that repeat head CT scans do not show progressive hemorrhagic injury (PHI) within the first 24 h. Recently, Spano et al. systematically reviewed 17 studies and found the rates of PHI to vary between 0% and 47%, but their recommendations are that anticoagulants reduce VTE without a corresponding increase in PHI. The majority of providers initiated anticoagulation within 24–72 h and a minority also initiated them within the first 24 h without demonstrating PHI.[
The successful protocol by Tignanelli et al. stratified patients according to their risk of TBI progression using the modified Berne-Norwood criteria and accordingly used prophylactic anticoagulation for low- and medium-risk patients.[
LIMITATIONS OF THIS REVIEW
This is a narrative review intended to provide a qualitative overview of the literature. Based on their subjective evaluations, the authors reviewed the literature and cited relevant articles. Despite the fact that this method is comprehensive, its nonsystematic nature is prone to bias. Robust deductions are limited due to a lack of quantitative synthesis of data from included studies. High-quality RCTs and systematic reviews of sTBI neurocritical care are limited in the literature. Due to the methodological limitations of narrative reviews, readers should cautiously interpret the conclusions brought forward by the authors of this study. Finally, although this review tackled several research questions that were proposed by the 2022 National Trauma Research Action Plan Neurotrauma Research Panel Delphi Survey,[
CONCLUSION
Severe TBI is a multifaceted disease process that requires diligent critical care to improve outcomes. SMPs are integral to achieve this. Repeated clinical examination should be used to detect critical neuroworsening, but multimodality neuromonitoring may be needed in select cases. Individualized ICP management may lead to better outcomes. The use of dynamic cerebrovascular reactivity indices such as the PRx may help achieve this. Hypertonic saline has theoretical advantages over mannitol as an ICP-lowering measure for IHT but supporting evidence is sparse. Although DC can be used to lower mortality for refractory IHT, poor functional outcomes limit its universal application for all patients. ICP monitoring continues to be a standard of care that likely improves outcomes. However, high-quality evidence should still be sought to define patients that stand to benefit most especially in pediatric age groups. The choice of device and timing of monitor placement are a matter of debate. Prophylaxis against seizures and VTE should be considered in SMPs but careful consideration is needed in patient selection for anti-seizure and anticoagulant prophylaxis. Beta-blockers can be added to SMPs to improve outcomes through prevention of PSH and posttraumatic hyperthermia. Amantadine is another promising neuroprotective agent that may improve cognitive recovery of sTBI patients. In spite of the consolidated research efforts in the refinement of SMPs, there are still many unanswered questions and novel research opportunities. We encourage future well-designed trials with clearly defined endpoints relevant to optimal patient care.
Authors’ contributions
STE conceived the idea and contributed to designing the review. MK participated in data extraction from the literature and drafting the manuscript. SHA participated in data extraction from the literature and drafting the manuscript. BB participated in data extraction from the literature and drafting the manuscript. MAR participated in data extraction from the literature and critical review. AA participated in data extraction from the literature and critical review. AEMA participated in data extraction from the literature and critical review. PWB participated in data extraction from the literature and critical review. AKB designed the review and performed critical revision of the manuscript. STE, MK, and AKB designed the algorithm.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation
Conflicts of interest
There are no conflicts of interest.
References
1. Aiolfi A, Benjamin E, Khor D, Inaba K, Lam L, Demetriades D. Brain trauma foundation guidelines for intracranial pressure monitoring: Compliance and effect on outcome. World J Surg. 2017. 41: 1543-9
2. Aiolfi A, Khor D, Cho J, Benjamin E, Inaba K, Demetriades D. Intracranial pressure monitoring in severe blunt head trauma: Does the type of monitoring device matter?. J Neurosurg. 2018. 128: 828-33
3. Åkerlund CA, Donnelly J, Zeiler FA, Helbok R, Holst A, Cabeleira M. Impact of duration and magnitude of raised intracranial pressure on outcome after severe traumatic brain injury: A CENTER-TBI high-resolution group study. PLoS One. 2020. 15: e0243427
4. Al Saiegh F, Philipp L, Mouchtouris N, Chalouhi N, Khanna O, Shah SO. Comparison of outcomes of severe traumatic brain injury in 36,929 patients treated with or without intracranial pressure monitoring in a mature trauma system. World Neurosurg. 2020. 136: e535-41
5. Alali AS, Fowler RA, Mainprize TG, Scales DC, Kiss A, De Mestral C. Intracranial pressure monitoring in severe traumatic brain injury: Results from the American College of surgeons trauma quality improvement program. J Neurotrauma. 2013. 30: 1737-46
6. Alali AS, McCredie VA, Mainprize TG, Gomez D, Nathens AB. Structure, process, and culture of intensive care units treating patients with severe traumatic brain injury: Survey of centers participating in the American college of surgeons trauma quality improvement program. J Neurotrauma. 2017. 34: 2760-7
7. Alkhoury F, Kyriakides TC. Intracranial pressure monitoring in children with severe traumatic brain injury: National trauma data bank-based review of outcomes. JAMA Surg. 2014. 149: 544-8
8. Allen A, Grigorian A, Christian A, Schubl SD, Barrios C, Lekawaet M. Intracranial pressure monitors associated with increased venous thromboembolism in severe traumatic brain injury. Eur J Trauma Emerg Surg. 2021. 47: 1483-90
9. Allen BB, Chiu YL, Gerber LM, Ghajar J, Greenfield JP. Age-specific cerebral perfusion pressure thresholds and survival in children and adolescents with severe traumatic brain injury*. Pediatr Crit Care Med. 2014. 15: 62-70
10. Asmar S, Bible L, Chehab M, Tang A, Khurrum M, Castanon L. Traumatic brain injury induced temperature dysregulation: What is the role of β blockers?. J Trauma Acute Care Surg. 2021. 90: 177-84
11. Baguley IJ, Perkes IE, Fernandez-Ortega JF, Rabinstein AA, Dolce G, Hendricks HT. Paroxysmal sympathetic hyperactivity after acquired brain injury: Consensus on conceptual definition, nomenclature, and diagnostic criteria. J Neurotrauma. 2014. 31: 1515-20
12. Balakrishnan B, Zhang L, Simpson PM, Hanson SJ. Impact of the timing of placement of an intracranial pressure monitor on outcomes in children with severe traumatic brain injury. Pediatr Neurosurg. 2018. 53: 379-86
13. Bales JW, Bonow RH, Buckley RT, Barber J, Temkin N, Chesnut RM. Primary external ventricular drainage catheter versus intraparenchymal ICP monitoring: Outcome analysis. Neurocrit Care. 2019. 31: 11-21
14. Benjamin E, Recinos G, Aiolfi A, Inaba K, Demetriades D. Pharmacological thromboembolic prophylaxis in traumatic brain injuries: Low molecular weight heparin is superior to unfractionated heparin. Ann Surg. 2017. 266: 463-9
15. Bennett TD, DeWitt PE, Greene TH, Srivastava R, Riva-Cambrin J, Nance ML. Functional outcome after intracranial pressure monitoring for children with severe traumatic brain injury. JAMA Pediatr. 2017. 171: 965-71
16. Byrne JP, Witiw CD, Schuster JM, Pascual JL, Cannon JW, Martin ND. Association of venous thromboembolism prophylaxis after neurosurgical intervention for traumatic brain injury with thromboembolic complications, repeated neurosurgery, and mortality. JAMA Surg. 2021. 157: 215794
17. Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ.editors. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017. 80: 6-15
18. Chen H, Song Z, Dennis JA. Hypertonic saline versus other intracranial pressure-lowering agents for people with acute traumatic brain injury. Cochrane Database Syst Rev. 2019. 12: CD010904
19. Chesnut R, Aguilera S, Buki A, Bulger E, Citerio G, Cooper DJ. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: The Seattle international severe traumatic brain injury consensus conference (SIBICC). Intensive Care Med. 2020. 46: 919-29
20. Chesnut RM, Temkin N, Dikmen S, Rondina C, Videtta W, Petroni G. A method of managing severe traumatic brain injury in the absence of intracranial pressure monitoring: The imaging and clinical examination protocol. J Neurotrauma. 2018. 35: 54-63
21. Chesnut RM, Temkin N, Videtta W, Petroni G, Lujan S, Pridgeon J. Consensus-based management protocol (CREVICE Protocol) for the treatment of severe traumatic brain injury based on imaging and clinical examination for use when intracranial pressure monitoring is not employed. J Neurotrauma. 2020. 37: 1291-9
22. Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, Ponsford J. Patient outcomes at twelve months after early decompressive craniectomy for diffuse traumatic brain injury in the randomized DECRA clinical trial. J Neurotrauma. 2020. 37: 810-6
23. De Silva MJ, Roberts I, Perel P, Edwards P, Kenward MG, Fernandes J. Patient outcome after traumatic brain injury in high-middle-and low-income countries: Analysis of data on 8927 patients in 46 countries. Int J Epidemiol. 2009. 38: 452-8
24. Delaplain PT, Grigorian A, Lekawa M, Mallicote M, Joe V, Schubl SD. Intracranial pressure monitoring associated with increased mortality in pediatric brain injuries. Pediatr Surg Int. 2020. 36: 391-8
25. Depreitere B, Citerio G, Smith M, Adelson PD, Aries MJ, Bleck TP. Cerebrovascular autoregulation monitoring in the management of adult severe traumatic brain injury: A delphi consensus of clinicians. Neurocrit Care. 2021. 34: 731-8
26. Ding H, Liao L, Zheng X, Wang Q, Liu Z, Xu G. β-blockers for traumatic brain injury: A systematic review and meta-analysis. J Trauma Acute Care Surg. 2021. 90: 1077-85
27. Donnelly J, Czosnyka M, Adams H, Cardim D, Kolias AG, Zeiler FA. Twenty-five years of intracranial pressure monitoring after severe traumatic brain injury: A retrospective, single-center analysis. Neurosurgery. 2019. 85: E75-82
28. Donnelly J, Smielewski P, Adams H, Zeiler FA, Cardim D, Liu X. Observations on the cerebral effects of refractory intracranial hypertension after severe traumatic brain injury. Neurocrit Care. 2020. 32: 437-47
29. Du J, Deng Y, Li H, Qiao S, Yu M, Xu Q. Ratio of optic nerve sheath diameter to eyeball transverse diameter by ultrasound can predict intracranial hypertension in traumatic brain injury patients: A prospective study. Neurocrit Care. 2020. 32: 478-85
30. Fang T, Valdes E, Frontera JA. Levetiracetam for seizure prophylaxis in neurocritical care: A systematic review and meta-analysis. Neurocrit Care. 2022. 36: 248-58
31. Ferguson PL, Smith GM, Wannamaker BB, Thurman DJ, Pickelsimer EE, Selassie AW. A population-based study of risk of epilepsy after hospitalization for traumatic brain injury. Epilepsia. 2010. 51: 891-8
32. Fletcher-Sandersjöö A, Thelin EP, Maegele M, Svensson M, Bellander BM. Time course of hemostatic disruptions after traumatic brain injury: A systematic review of the literature. Neurocrit Care. 2021. 34: 635-56
33. Frey LC. Epidemiology of posttraumatic epilepsy: A critical review. Epilepsia. 2003. 44: 11-17
34. Ganslandt O, Mourtzoukos S, Stadlbauer A, Sommer B, Rammensee R. Evaluation of a novel noninvasive ICP monitoring device in patients undergoing invasive ICP monitoring: Preliminary results. J Neurosurg. 2018. 128: 1653-60
35. Garvin R, Mangat HS. Emergency neurological life support: Severe traumatic brain injury. Neurocrit Care. 2017. 27: 159-69
36. Godoy DA, Lubillo S, Rabinstein AA. Pathophysiology and management of intracranial hypertension and tissular brain hypoxia after severe traumatic brain injury: An integrative approach. Neurosurg Clin N Am. 2018. 29: 195-212
37. Gu J, Huang H, Huang Y, Sun H, Xu H. Hypertonic saline or mannitol for treating elevated intracranial pressure in traumatic brain injury: A meta-analysis of randomized controlled trials. Neurosurg Rev. 2019. 42: 499-509
38. Hasen M, Gomez A, Froese L, Dian J, Raj R, Thelin EP. Alternative continuous intracranial pressure-derived cerebrovascular reactivity metrics in traumatic brain injury: A scoping overview. Acta Neurochir (Wien). 2020. 162: 1647-62
39. Hawryluk GW, Manley GT. Classification of traumatic brain injury: Past, present, and future. Handb Clin Neurol. 2015. 127: 15-21
40. Hawryluk GW, Aguilera S, Buki A, Bulger E, Citerio G, Cooper DJ. A management algorithm for patients with intracranial pressure monitoring: The Seattle international severe traumatic brain injury consensus conference (SIBICC). Intensive Care Med. 2019. 45: 1783-94
41. Hawryluk GW, Rubiano AM, Totten AM, O’Reilly C, Ullman JS, Bratton SL. Guidelines for the management of severe traumatic brain injury: 2020 update of the decompressive craniectomy recommendations. Neurosurgery. 2020. 87: 427-34
42. Hoffman H, Abi-Aad K, Bunch KM, Beutler T, Otite FO, Chin LS. Outcomes associated with brain tissue oxygen monitoring in patients with severe traumatic brain injury undergoing intracranial pressure monitoring. J Neurosurg. 2021. 135: 1799-806
43. Hoffman H, Bunch KM, Protas M, Chin LS. The effect of timing of intracranial pressure monitor placement in patients with severe traumatic brain injury. Neurocrit Care. 2021. 34: 167-74
44. Huijben JA, Wiegers EJ, De Keizer NF, Maas AI, Menon D, Ercole A. Development of a quality indicator set to measure and improve quality of ICU care for patients with traumatic brain injury. Crit Care. 2019. 23: 95
45. Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016. 375: 1119-30
46. Katz DI, Bernick C, Dodick DW, Mez J, Mariani ML, Adleret CH. National institute of neurological disorders and stroke consensus diagnostic criteria for traumatic encephalopathy syndrome. Neurology. 2021. 96: 848-63
47. Khalili H, Ahl R, Paydar S, Sjolin G, Cao Y, Fard HA. Beta-blocker therapy in severe traumatic brain injury: A prospective randomized controlled trial. World J Surg. 2020. 44: 1844-53
48. Kim H, Lee HJ, Kim YT, Son Y, Smielewski P, Czosnykaet M. Novel index for predicting mortality during the first 24 hours after traumatic brain injury. J Neurosurg. 2018. 131: 1887-95
49. Komisarow JM, Toro C, Curley J, Mills B, Cho C, Simo GM. Utilization of brain tissue oxygenation monitoring and association with mortality following severe traumatic brain injury. Neurocrit Care. 2022. 36: 350-6
50. Kramer AH, Couillard PL, Zygun DA, Aries MJ, Gallagher CN. Continuous assessment of “optimal” cerebral perfusion pressure in traumatic brain injury: A cohort study of feasibility, reliability, and relation to outcome. Neurocrit Care. 2019. 30: 51-61
51. Kramer AH, Zygun DA. Neurocritical care: Why does it make a difference?. Curr Opin Crit Care. 2014. 20: 174-81
52. Krishnamoorthy V, Temkin N, Barber J, Foreman B, Komisarow J, Korley FK. Association of early multiple organ dysfunction with clinical and functional outcomes over the year following traumatic brain injury: A transforming research and clinical knowledge in traumatic brain injury study. Crit Care Med. 2021. 49: 1769-78
53. Laroche M, Kutcher ME, Huang MC, Cohen MJ, Manley GT. Coagulopathy after traumatic brain injury. Neurosurgery. 2012. 70: 1334-45
54. Lazaridis C, DeSantis SM, Smielewski P, Menon DK, Hutchinson P, Pickard JD. Patient-specific thresholds of intracranial pressure in severe traumatic brain injury. J Neurosurg. 2014. 120: 893-900
55. Lazaridis C, Robertson CS. The role of multimodal invasive monitoring in acute traumatic brain injury. Neurosurg Clin N Am. 2016. 27: 509-17
56. Lazaridis C, Smielewski P, Menon DK, Hutchinson P, Pickard JD, Czosnyka M. Patient-specific thresholds and doses of intracranial hypertension in severe traumatic brain injury. Acta Neurochir Suppl. 2016. 122: 117-20
57. Le Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM. Consensus summary statement of the international multidisciplinary consensus conference on multimodality monitoring in neurocritical care: A statement for healthcare professionals from the neurocritical care society and the European society of intensive care medicine. Intensive Care Med. 2014. 40: 1189-209
58. Ley EJ, Brown CV, Moore EE, Sava JA, Peck K, Ciesla DJ. Updated guidelines to reduce venous thromboembolism in trauma patients: A western trauma association critical decisions algorithm. J Trauma Acute Care Surg. 2020. 89: 971-81
59. Liu H, Wang W, Cheng F, Yuan Q, Yang J, Hu J. External ventricular drains versus intraparenchymal intracranial pressure monitors in traumatic brain injury: A prospective observational study. World Neurosurg. 2015. 83: 794-800
60. Lubillo ST, Parrilla DM, Blanco J, Morera J, Dominguez J, Belmonte F. Prognostic value of changes in brain tissue oxygen pressure before and after decompressive craniectomy following severe traumatic brain injury. J Neurosurg. 2018. 128: 1538-46
61. Makarenko S, Griesdale DE, Gooderham P, Sekhon MS. Multimodal neuromonitoring for traumatic brain injury: A shift towards individualized therapy. J Clin Neurosci. 2016. 26: 8-13
62. Manfiotto M, Beccaria K, Rolland A, Paternoster G, Plas B, Boetto S. Decompressive craniectomy in children with severe traumatic brain injury: A multicenter retrospective study and literature review. World Neurosurg. 2019. 129: e56-62
63. Mangat HS, Wu X, Gerber LM, Schwarz JT, Fakhar M, Murthy SB. Hypertonic saline is superior to mannitol for the combined effect on intracranial pressure and cerebral perfusion pressure burdens in patients with severe traumatic brain injury. Neurosurgery. 2020. 86: 221-30
64. Margolick J, Dandurand C, Duncan K, Chen W, Evans DC, Sekhon MS. A systematic review of the risks and benefits of venous thromboembolism prophylaxis in traumatic brain injury. Can J Neurol Sci. 2018. 45: 432-44
65. Marklund N. The neurological wake-up test-a role in neurocritical care monitoring of traumatic brain injury patients?. Front Neurol. 2017. 8: 540
66. Marshall LF, Marshall SB, Klauber MR, Clark MV, Eisenberg H, Jane JA. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992. 9: S287-92
67. Matsushima K, Leichtle SW, Wild J, Young K, Chang G, Demetriades D. Anticoagulation therapy in patients with traumatic brain injury: An eastern association for the surgery of trauma multicenter prospective study. Surgery. 2021. 169: 470-6
68. McCredie VA, Alali AS, Scales DC, Rubenfeld GD, Cuthbertson BH, Nathens AB. Impact of ICU structure and processes of care on outcomes after severe traumatic brain injury: A multicenter cohort study. Crit Care Med. 2018. 46: 1139-49
69. Mohamed MS, El Sayed I, Zaki A, Abdelmonem S. Assessment of the effect of amantadine in patients with traumatic brain injury: A meta-analysis. J Trauma Acute Care Surg. 2022. 92: 605-14
70. Narayan V, Mohammed N, Savardekar AR, Patra DP, Notarianni C, Nanda A. Noninvasive intracranial pressure monitoring for severe traumatic brain injury in children: A concise update on current methods. World Neurosurg. 2018. 114: 293-300
71. Needham E, McFadyen C, Newcombe V, Synnot AJ, Czosnyka M, Menon D. Cerebral perfusion pressure targets individualized to pressure-reactivity index in moderate to severe traumatic brain injury: A systematic review. J Neurotrauma. 2017. 34: 963-70
72. Ng SY, Lee AY. Traumatic brain injuries: Pathophysiology and potential therapeutic targets. Front Cell Neurosci. 2019. 13: 528
73. Okonkwo DO, Shutter LA, Moore C, Temkin NR, Puccio AM, Madden CJ. Brain oxygen optimization in severe traumatic brain injury Phase-II: A Phase II randomized trial. Crit Care Med. 2017. 45: 1907-14
74. Patil H, Gupta R. A comparative study of bolus dose of hypertonic saline, mannitol, and mannitol plus glycerol combination in patients with severe traumatic brain injury. World Neurosurg. 2019. 125: e221-8
75. Piccinini A, Lewis M, Benjamin E, Aiolfi A, Inaba K, Demetriades D. Intracranial pressure monitoring in severe traumatic brain injuries: A closer look at Level 1 trauma centers in the United States. Injury. 2017. 48: 1944-50
76. Podell JE, Miller SS, Jaffa MN, Pajoumand M, Armahizer M, Chen H. Admission features associated with paroxysmal sympathetic hyperactivity after traumatic brain injury: A case-control study. Crit Care Med. 2021. 49: e989-1000
77. Rangel-Castilla L, Gasco J, Nauta HJ, Okonkwo DO, Robertson CS. Cerebral pressure autoregulation in traumatic brain injury. Neurosurg Focus. 2008. 25: E7
78. Rappold JF, Sheppard FR, Carmichael Ii SP, Cuschieri J, Ley E, Rangel E. Venous thromboembolism prophylaxis in the trauma intensive care unit: An American association for the surgery of trauma critical care committee clinical consensus document. Trauma Surg Acute Care Open. 2021. 6: e000643
79. Rasulo FA, Bertuetti R, Robba C, Lusenti F, Cantoni A, Bernini M. The accuracy of transcranial Doppler in excluding intracranial hypertension following acute brain injury: A multicenter prospective pilot study. Crit Care. 2017. 21: 44
80. Reiff DA, Haricharan RN, Bullington NM, Griffin RL, McGwin G, Rue LW. Traumatic brain injury is associated with the development of deep vein thrombosis independent of pharmacological prophylaxis. J Trauma. 2009. 66: 1436-40
81. Riemann L, Beqiri E, Younsi A, Czosnyka M, Smielewski P. Predictive and discriminative power of pressure reactivity indices in traumatic brain injury. Neurosurgery. 2020. 87: 655-63
82. Robba C, Graziano F, Rebora P, Elli F, Giussani PC, Oddo PM. Intracranial pressure monitoring in patients with acute brain injury in the intensive care unit (SYNAPSE-ICU): An international, prospective observational cohort study. Lancet Neurol. 2021. 20: 548-58
83. Robba C, Pozzebon S, Moro B, Vincent JL, Creteur J, Taccone FS. Multimodal non-invasive assessment of intracranial hypertension: An observational study. Crit Care. 2020. 24: 379
84. Roldán M, Abay TY, Kyriacou PA. Non-invasive techniques for multimodal monitoring in traumatic brain injury: Systematic review and meta-analysis. J Neurotrauma. 2020. 37: 2445-53
85. Rønning P, Helseth E, Skaga NO, Stavem K, Langmoen IA. The effect of ICP monitoring in severe traumatic brain injury: A propensity score-weighted and adjusted regression approach. J Neurosurg. 2018. 131: 1896-904
86. Rosenthal G, Sanchez-Mejia RO, Phan N, Hemphill JC, Martin C, Manley GT. Incorporating a parenchymal thermal diffusion cerebral blood flow probe in bedside assessment of cerebral autoregulation and vasoreactivity in patients with severe traumatic brain injury. J Neurosurg. 2011. 114: 62-70
87. Shen L, Wang Z, Su Z, Qiu S, Xu J, Zhou Y. Effects of intracranial pressure monitoring on mortality in patients with severe traumatic brain injury: A meta-analysis. PLoS One. 2016. 11: e0168901
88. Sorrentino E, Diedler J, Kasprowicz M, Budohoski KP, Haubrich C, Smielewski P. Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit Care. 2012. 16: 258-66
89. Spano PJ, Shaikh S, Boneva D, Hai S, McKenney M, Elkbuli A. Anticoagulant chemoprophylaxis in patients with traumatic brain injuries: A systematic review. J Trauma Acute Care Surg. 2020. 88: 454-60
90. Stein DM, Braverman MA, Phuong J, Shipper E, Price MA, Bixby PJ. Developing a national trauma research action plan (NTRAP): Results from the neurotrauma research panel delphi Survey. J Trauma Acute Care Surg. 2022. 92: 906-15
91. Stocchetti N, Carbonara M, Citerio G, Ercole A, Skrifvars MB, Smielewski P. Severe traumatic brain injury: Targeted management in the intensive care unit. Lancet Neurol. 2017. 16: 452-64
92. Tavakoli S, Peitz G, Ares W, Hafeez S, Grandhi R. Complications of invasive intracranial pressure monitoring devices in neurocritical care. Neurosurg Focus. 2017. 43: E6
93. Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths-United States, 2007 and 2013. MMWR Surveill Summ. 2017. 66: 1-16
94. The Management and Rehabilitation of Post-Acute Mild Traumatic Brain Injury Work Group. VA/DoD Clinical Practice Guideline for the Management and Rehabilitation of Post-Acute Mild Traumatic Brain Injury. Available from: https://www.healthquality.va.gov/guidelines/rehab/mtbi [Last accessed on 2021 Jun 21].
95. Tignanelli CJ, Gipson J, Nguyen A, Martinez R, Yang S, Reicks PL. Implementation of a prophylactic anticoagulation guideline for patients with traumatic brain injury. Jt Comm J Qual Patient Saf. 2020. 46: 185-91
96. Truong EI, Stanley SP, DeMario BS, Tseng ES, Como JJ, Ho VP. Variation in neurosurgical intervention for severe traumatic brain injury: The challenge of measuring quality in trauma center verification. J Trauma Acute Care Surg. 2021. 91: 114-20
97. Van Erp IA, Gaitanidis A, El Moheb M, Kaafarani HM, Saillant N, Duhaime AC. Low-molecular-weight heparin versus unfractionated heparin in pediatric traumatic brain injury. J Neurosurg Pediatr. 2021. 27: 469-74
98. Varsos GV, Kolias AG, Smielewski P, Brady KM, Varsos VG, Hutchinsonet PJ. A noninvasive estimation of cerebral perfusion pressure using critical closing pressure. J Neurosurg. 2015. 123: 638-48
99. Verellen RM, Cavazos JE. Post-traumatic epilepsy: An overview. Therapy. 2010. 7: 527-31
100. Volovici V, Huijben JA, Ercole A, Stocchetti N, Dirven CM, Van Der Jagt M. Ventricular drainage catheters versus intracranial parenchymal catheters for intracranial pressure monitoring-based management of traumatic brain injury: A systematic review and meta-analysis. J Neurotrauma. 2019. 36: 988-95
101. Wat R, Mammi M, Paredes J, Haines J, Alasmari M, Liew A. The effectiveness of antiepileptic medications as prophylaxis of early seizure in patients with traumatic brain injury compared with placebo or no treatment: A systematic review and meta-analysis. World Neurosurg. 2019. 122: 433-40
102. Wilson L, Stewart W, Dams-O’Connor K, Diaz-Arrastia R, Horton L, Menon DK. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017. 16: 813-25
103. Woods KS, Horvat CM, Kantawala S, Simon DW, Rakkar J, Kochanek PM. Intracranial and cerebral perfusion pressure thresholds associated with inhospital mortality across pediatric neurocritical care. Pediatr Crit Care Med. 2021. 22: 135-46
104. Xu JC, Shen J, Shao WZ, Tang LJ, Sun YZ, Zhai XF. The safety and efficacy of levetiracetam versus phenytoin for seizure prophylaxis after traumatic brain injury: A systematic review and meta-analysis. Brain Inj. 2016. 30: 1054-61
105. Yuan Q, Wu X, Cheng H, Yang C, Wang Y, Wang E. Is intracranial pressure monitoring of patients with diffuse traumatic brain injury valuable? An observational multicenter study. Neurosurgery. 2016. 78: 361-8
106. Yuan Q, Wu X, Sun Y, Yu J, Li Z, Du Z. Impact of intracranial pressure monitoring on mortality in patients with traumatic brain injury: A systematic review and meta-analysis. J Neurosurg. 2015. 122: 574-87
107. Zeiler FA, Beqiri E, Cabeleira M, Hutchinson PJ, Stocchetti N, Menonet DK. Brain tissue oxygen and cerebrovascular reactivity in traumatic brain injury: A collaborative European neurotrauma effectiveness research in traumatic brain injury exploratory analysis of insult burden. J Neurotrauma. 2020. 37: 1854-63
108. Zeiler FA, Donnelly J, Calviello L, Smielewski P, Menon DK, Czosnyka M. Pressure autoregulation measurement techniques in adult traumatic brain injury, Part II: A scoping review of continuous methods. J Neurotrauma. 2017. 34: 3224-37
109. Zeiler FA, Donnelly J, Smielewski P, Menon DK, Hutchinson PJ, Czosnyka M. Critical thresholds of intracranial pressure-derived continuous cerebrovascular reactivity indices for outcome prediction in noncraniectomized patients with traumatic brain injury. J Neurotrauma. 2018. 35: 1107-15
110. Zeiler FA, Ercole A, Cabeleira M, Beqiri E, Zoerle T, Carbonara M. Patient-specific ICP epidemiologic thresholds in adult traumatic brain injury: A CENTER-TBI validation study. J Neurosurg Anesthesiol. 2021. 33: 28-38







Imara Hernandez
Posted January 18, 2023, 4:01 pm
Excelente revision,aclarando el término de neuroempeoramiento crítico y su diagnóstico precoz.