- Department of Neurosurgery, Fukui Prefectural Hospital, Fukui, Japan.
Correspondence Address:
Katsuyoshi Miyashita, Department of Neurosurgery, Fukui Prefectural Hospital, Fukui, Japan.
DOI:10.25259/SNI_18_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Katsuyoshi Miyashita, Kosuke Nambu, Shio Kinami, Kenshu Nogami, Iku Nambu, Yasuo Tohma. Intra-and peritumoral hemorrhage in the meningioma of a nonagenarian due to administration of direct oral anticoagulants after mechanical thrombectomy. 05-May-2023;14:164
How to cite this URL: Katsuyoshi Miyashita, Kosuke Nambu, Shio Kinami, Kenshu Nogami, Iku Nambu, Yasuo Tohma. Intra-and peritumoral hemorrhage in the meningioma of a nonagenarian due to administration of direct oral anticoagulants after mechanical thrombectomy. 05-May-2023;14:164. Available from: https://surgicalneurologyint.com/surgicalint-articles/12311/
Abstract
Background: Spontaneous intratumoral hemorrhage of meningiomas is rare, and their incidence due to anticoagulants is unclear. The incidence of both meningioma and cardioembolic stroke increases with age. We report the very elderly case of intra- and peritumoral hemorrhage in frontal meningioma induced by direct oral anticoagulant (DOAC) following mechanical thrombectomy, in whom, surgical resection was required 10 years after the tumor was first indicated.
Case Description: A 94-year-old woman with independence in daily living who suffered sudden consciousness disturbance, total aphasia, and right hemiparesis was admitted to our hospital. Magnetic resonance imaging showed an acute cerebral infarction and left middle cerebral artery occlusion. There was also a left frontal meningioma with peritumoral edema, which was discovered 10 years prior, and the size and edema had remarkably increased. The patient underwent urgent mechanical thrombectomy, and recanalization was achieved. Administration of DOAC was initiated for the atrial fibrillation. Computed tomography (CT) revealed an asymptomatic intratumoral hemorrhage at postoperative day 26. The patient’s symptoms gradually improved; however, she suffered sudden disturbance of consciousness and right hemiparesis on postoperative day 48. CT revealed intra- and peritumoral hemorrhages with compression of the surrounding brain. Therefore, we decided to perform tumor resection instead of conservative treatment. The patient underwent surgical resection, and the postoperative course was uneventful. It was diagnosed with transitional meningioma with no malignant features. The patient was transferred to another hospital for rehabilitation.
Conclusion: Peritumoral edema representing a pial blood supply might be a significant factor associated with intracranial hemorrhage due to DOAC administration in patients with meningioma. The evaluation of hemorrhagic risk due to DOACs is important not only for meningioma but also for other brain tumor cases.
Keywords: Direct oral anticoagulant, Intratumoral hemorrhage, Mechanical thrombectomy, Meningioma
INTRODUCTION
The incidence of atrial fibrillation increases with age; therefore, that of cardioembolic stroke in elderly patients also increases. It has been reported that the incidence of cardioembolic stroke sharply increases with age, especially in those aged >80 years.[
The incidence of meningioma also increases with age. It was reported that its in patients over 85 years of age doubled from the ages of 65–69 years.[
Here, we report on an intra-and peritumoral hemorrhage in the frontal meningioma of a very elderly patient induced by DOAC administration following mechanical thrombectomy, and surgical resection was required 10 years after the tumor was revealed.
CASE REPORT
A 94-year-old woman with independence in daily living who suffered sudden consciousness disturbance and right hemiparesis was admitted to our hospital. Magnetic resonance imaging (MRI) revealed an acute cerebral infarction in the left corona radiata and a left middle cerebral artery occlusion [
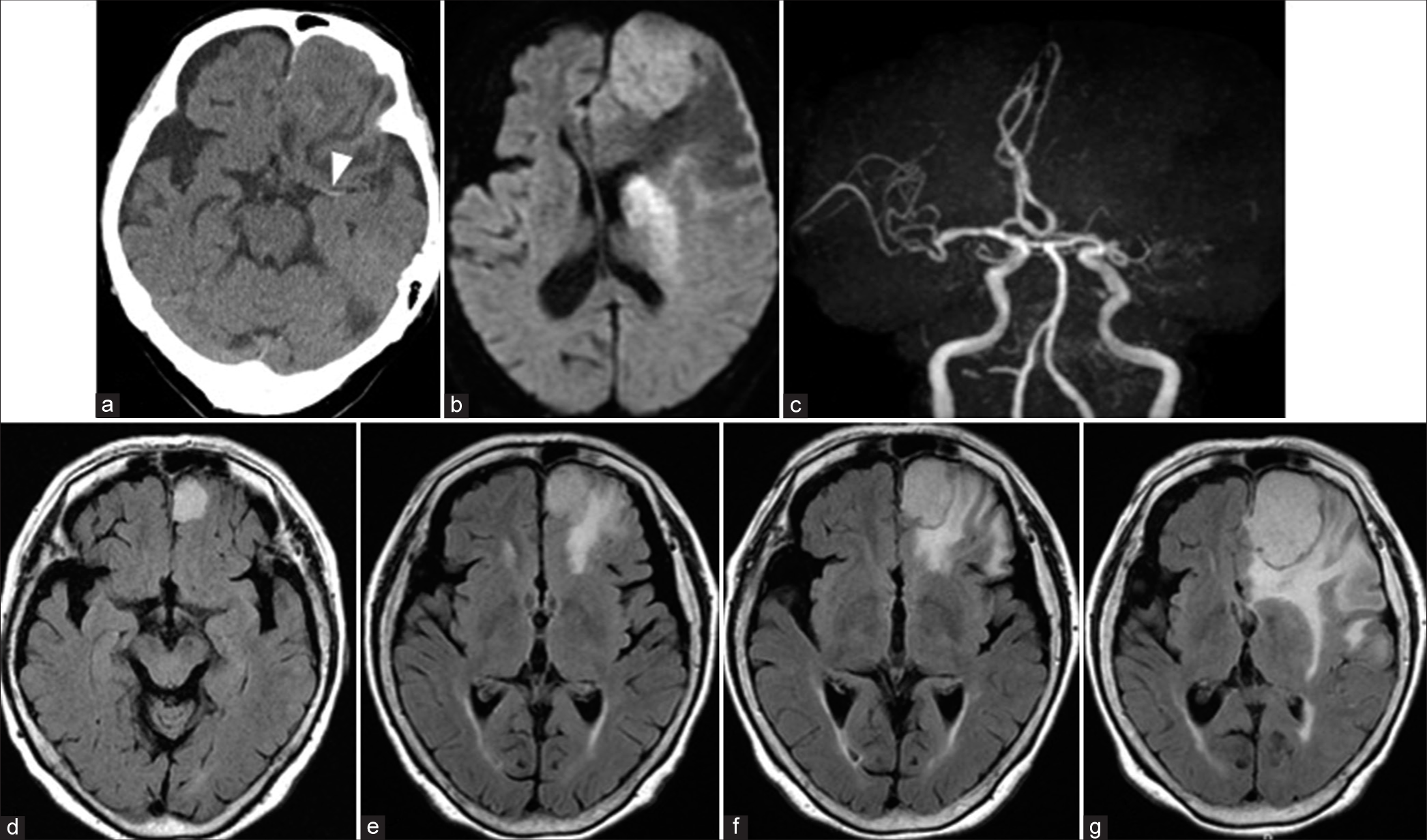
Figure 1:
Preoperative computed tomography (CT) and magnetic resonance imaging (MRI). CT showing a left frontal tumor and left hyper dense sign (arrowhead) (a). Diffusion-weighted image on MRI showing an acute cerebral infarction (b), and magnetic resonance angiography showing a left middle cerebral artery occlusion (c). Fluid-attenuated inversion recovery image on MRI showing enlargement of tumor and peritumoral edema 10 years prior (d), 7 years prior (e), 5 years prior (f) to the onset, and at the onset of cerebral infarction (g).
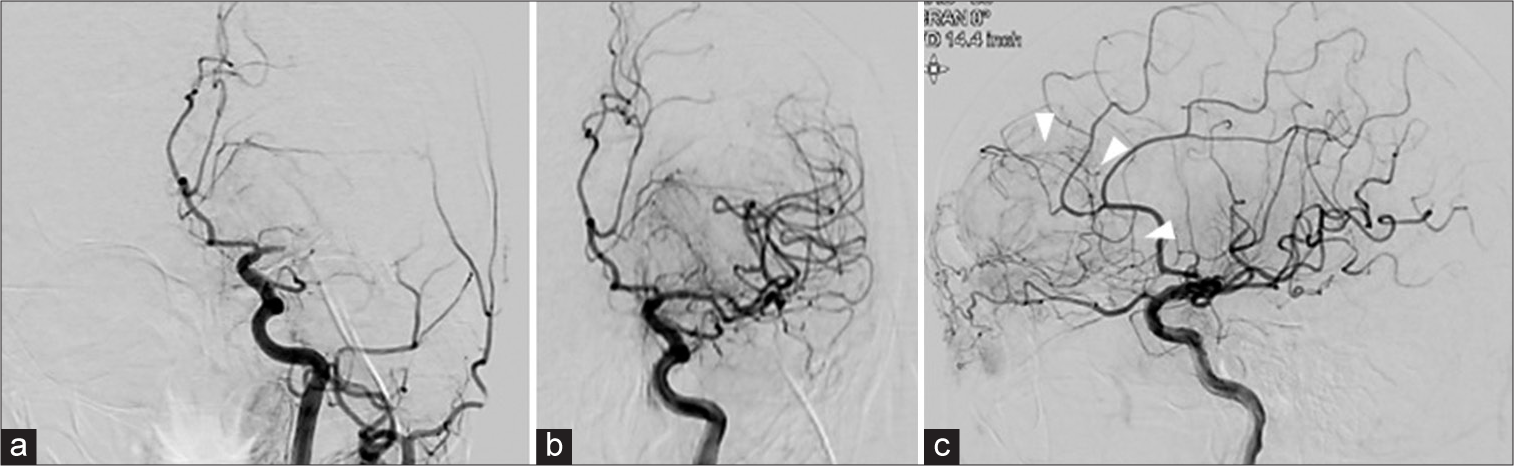
Figure 2:
Digital subtraction angiography (DSA) of the pre- and post-mechanical thrombectomy. Pre-DSA showing a left middle cerebral artery occlusion (a). Post-DSA showing the recanalization of the middle cerebral artery (b) and the tumor staining of left frontal tumor with a pial supply in the posterior part of the tumor (arrow head) (c).
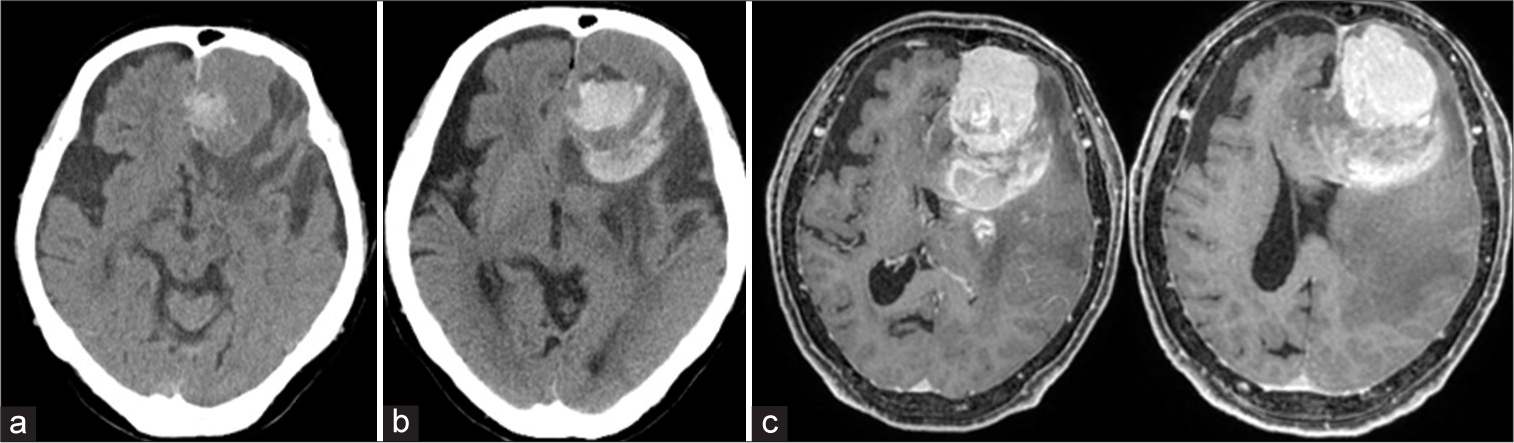
Computed tomography (CT) revealed an asymptomatic intratumoral hemorrhage at postoperative day 26 [
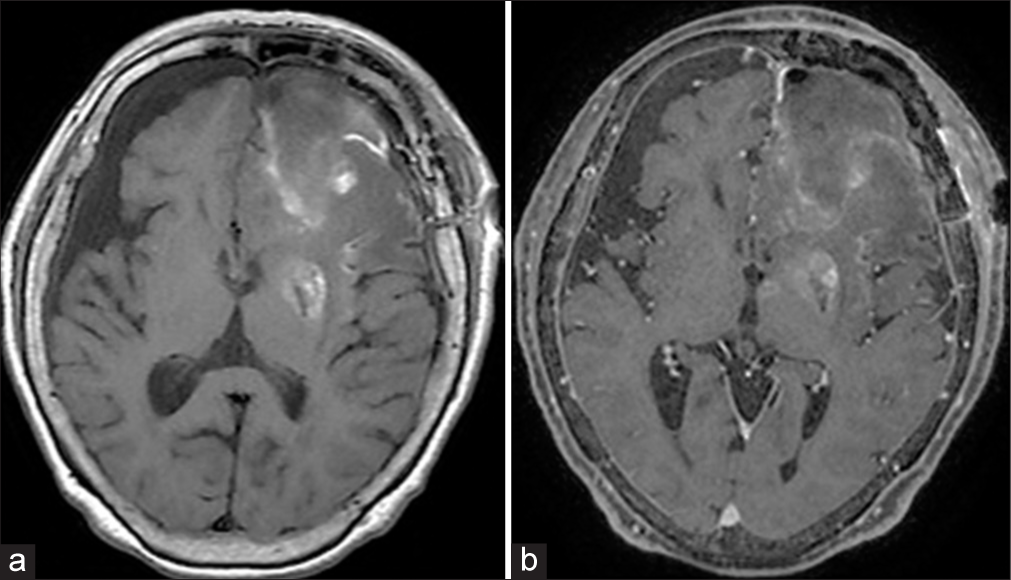
Figure 3:
Postoperative day 26 computed tomography (CT) showing intratumoral hemorrhage (a). Postoperative day 48 CT showing intra and peritumoral hemorrhage (b). Gadolinium-enhanced T1-weighted image on MRI showing a diffuse enhanced tumor with an intratumoral hemorrhage and peritumoral hemorrhage compressing the surrounding brain (c).
DISCUSSION
Several reports have mentioned that the incidence of ICH in patients with intracranial tumors did not increase with DOAC administration compared with low-molecular-weight heparin (LMWH) administration. In the primary brain tumors, the cumulative incidences of major ICH at 12 months with DOAC and LMWH administration was 0% and 18.2%, respectively.[
The mechanism of ICH associated with meningiomas remains unclear. In the present case, the most critical factor was peritumoral hemorrhage rather than intratumoral hemorrhage. Several reports have described peritumoral edema in meningiomas associated with a pial blood supply and expression of vascular endothelial growth factor.[
The appropriateness of surgical resection for nonagenarian meningiomas is debatable. Surgery for meningiomas over the age of 80 years is feasible when the SKALE score is over 8.[
CONCLUSION
We report the rare case of intra-and peritumoral hemorrhage due to DOAC administration in a very elderly meningioma patient following mechanical thrombectomy. Peritumoral edema representing a pial blood supply might be an important factor that increases the risk of ICH due to DOACs in meningioma cases. The incidence of both meningioma and cardioembolic stroke increases with age; thus, combined cases such as that in this this report may become more common in super-aged societies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Achey RL, Gittleman H, Schroer J, Khanna V, Kruchko C, Barnholtz-Sloan JS. Nonmalignant and malignant meningioma incidence and survival in the elderly, 2005-2015, using the Central Brain Tumor Registry of the United States. Neuro Oncol. 2019. 21: 380-91
2. Bitzer M, Wöckel L, Luft AR, Wakhloo AK, Petersen D, Opitz H. The importance of pial blood supply to the development of peritumoral brain edema in meningiomas. J Neurosurg. 1997. 87: 368-73
3. Bosnjak R, Derham C, Popović M, Ravnik J. Spontaneous intracranial meningioma bleeding: Clinicopathological features and outcome. J Neurosurg. 2005. 103: 473-84
4. Carney BJ, Uhlmann EJ, Puligandla M, Mantia C, Weber GM, Neuberg DS. Intracranial hemorrhage with direct oral anticoagulants in patients with brain tumors. J Thromb Haemost. 2019. 17: 72-6
5. Huang R, Su S, Yang Z, Wang H, Hong L, Chen L. Neuroradiologic findings and clinical features of meningiomas with spontaneous hemorrhagic onset: A single-center 10-year experience. World Neurosurg. 2022. 162: e605-15
6. Ito Y, Nakajima M, Watari M, Sakamoto T, Hashimoto Y, Tajiri S. Intratumoral hemorrhage in a patient with malignant meningioma under anticoagulant therapy. J Stroke Cerebrovasc Dis. 2015. 24: e91-2
7. Leader A, Hamulyak EN, Carney BJ, Avrahami M, Knip JJ, Rozenblatt S. Intracranial hemorrhage with direct oral anticoagulants in patients with brain metastases. Blood Adv. 2020. 4: 6291-7
8. Maeda K, Gotoh H, Chikui E, Furusawa T. Intratumoral hemorrhage from a posterior fossa tumor after cardiac valve surgery--case report. Neurol Med Chir (Tokyo). 2001. 41: 548-50
9. Miyajima Y, Oka H, Utsuki S, Shimizu S, Suzuki S, Fujii K. Intracranial hemorrhage associated with convexity meningioma after cerebral angiography--case report. Neurol Med Chir (Tokyo). 2010. 50: 67-70
10. Nakagawa T, Onozuka S, Mayanagi K. Two cases of hemorrhage in benign brain tumors during systemic heparinization. Case reports. Interv Neuroradiol. 2001. 7: 127-30
11. Pistolesi S, Fontanini G, Camacci T, De Ieso K, Boldrini L, Lupi G. Meningioma-associated brain oedema: The role of angiogenic factors and pial blood supply. J Neurooncol. 2002. 60: 159-64
12. Reed-Guy L, Desai AS, Phillips RE, Croteau D, Albright K, O’Neill M. Risk of intracranial hemorrhage with direct oral anticoagulants vs low molecular weight heparin in glioblastoma: A retrospective cohort study. Neuro Oncol. 2022. 24: 2172-9
13. Sacko O, Sesay M, Roux FE, Riem T, Grenier B, Liguoro D. Intracranial meningioma surgery in the ninth decade of life. Neurosurgery. 2007. 61: 950-954 discussion 955
14. Swartz AW, Drappatz J. Safety of direct oral anticoagulants in central nervous system malignancies. Oncologist. 2021. 26: 427-32
15. Yiin GS, Howard DP, Paul NL, Li L, Luengo-Fernandez R, Bullx LM. Age-specific incidence, outcome, cost, and projected future burden of atrial fibrillation-related embolic vascular events: A population-based study. Circulation. 2014. 130: 1236-44
16. Yoshioka H, Hama S, Taniguchi E, Sugiyama K, Arita K, Kurisu K. Peritumoral brain edema associated with meningioma: Influence of vascular endothelial growth factor expression and vascular blood supply. Cancer. 1999. 85: 936-44