- Department of Neurosurgery, Iwate Prefectural Ofunato Hospital, Ofunato,
- Department of Neurosurgery, Iwate Prefectural Chubu Hospital, Kitakami,
- Department of Neurosurgery, Iwate Medical University, Yahaba, Iwate, Japan.
Correspondence Address:
Sotaro Oshida, Department of Neurosurgery, Iwate Prefectural Ofunato Hospital, Ofunato, Iwate, Japan.
DOI:10.25259/SNI_1144_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sotaro Oshida1, Yosuke Akamatsu2, Yoshiyasu Matsumoto1, Taro Suzuki1, Takuto Sasaki1, Yuki Kondo1, Shunrou Fujiwara3, Hiroshi Kashimura2, Yoshitaka Kubo3, Kuniaki Ogasawara3. Intracranial aneurysm rupture within three days after receiving mRNA anti-COVID-19 vaccination: Three case reports. 31-Mar-2022;13:117
How to cite this URL: Sotaro Oshida1, Yosuke Akamatsu2, Yoshiyasu Matsumoto1, Taro Suzuki1, Takuto Sasaki1, Yuki Kondo1, Shunrou Fujiwara3, Hiroshi Kashimura2, Yoshitaka Kubo3, Kuniaki Ogasawara3. Intracranial aneurysm rupture within three days after receiving mRNA anti-COVID-19 vaccination: Three case reports. 31-Mar-2022;13:117. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11501
Abstract
Background: Although neurological adverse events have been reported after receiving coronavirus disease 2019 (COVID-19) vaccines, associations between COVID-19 vaccination and aneurysmal subarachnoid hemorrhage (SAH) have rarely been discussed. We report here the incidence and details of three patients who presented with intracranial aneurysm rupture shortly after receiving messenger ribonucleic acid (mRNA) COVID-19 vaccines.
Case Description: We retrospectively reviewed the medical records of individuals who received a first and/ or second dose of mRNA COVID-19 vaccine between March 6, 2021, and June 14, 2021, in a rural district in Japan, and identified the occurrences of aneurysmal SAH within 3 days after mRNA vaccination. We assessed incidence rates (IRs) for aneurysmal SAH within 3 days after vaccination and spontaneous SAH for March 6–June 14, 2021, and for the March 6–June 14 intervals of a 5-year reference period of 2013–2017. We assessed the incidence rate ratio (IRR) of aneurysmal SAH within 3 days after vaccination and spontaneous SAH compared to the crude incidence in the reference period (2013–2017). Among 34,475 individuals vaccinated during the study period, three women presented with aneurysmal SAH (IR: 1058.7/100,000 person-years), compared with 83 SAHs during the reference period (IR: 20.7/100,000 persons-years). IRR was 0.026 (95% confidence interval [CI] 0.0087–0.12; P P = 0.204). All three cases developed SAH within 3 days (range, 0–3 days) of the first or second dose of BNT162b2 mRNA COVID-19 vaccine by Pfizer/BioNTech. The median age at the time of SAH onset was 63.7 years (range, 44– 75 years). Observed locations of ruptured aneurysms in patients were the bifurcations of the middle cerebral artery, internal carotid-posterior communicating artery, and anterior communicating artery, respectively. Favorable outcomes (modified Rankin scale scores, 0–2) were obtained following microsurgical clipping or intra-aneurysm coiling.
Conclusion: Although the advantages of COVID-19 vaccination appear to outweigh the risks, pharmacovigilance must be maintained to monitor potentially fatal adverse events and identify possible associations.
Keywords: Adverse events, mRNA COVID-19 vaccine, Ruptured aneurysm, Subarachnoid hemorrhage
INTRODUCTION
Since late 2019, coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly and infected millions worldwide. The intensity and rapidity of SARS-CoV-2 transmission have led to substantial morbidity and mortality, putting considerable pressure on public health systems. Japan started administering vaccines to healthcare workers on February 17, 2021, and subsequently began a campaign of broad inoculation of the general public to ameliorate the effects of the pandemic.[
The Ministry of Health, Labour, and Welfare (MHLW) in Japan reported that t, as of May 7, 2021, a total of 39 people had died after receiving COVID-19 vaccinations.[
A previous systematic review[
CASE DESCRIPTION
We retrospectively reviewed the electronic medical records of all individuals who received a first and/or second dose of BNT162b2 mRNA COVID-19 vaccine between March 6, 2021 and June 14, 2021 (COVID-19 pandemic, 100 days), in the Kenou area (Kitakami City, Hanamaki City, Tono City, and Nishiwaga Town) and Kesen area (Ofunato City, Rikuzentakata City, and Sumita Town), both of which are rural districts in Iwate Prefecture, Japan. The reason for enrolling cases from March 6, 2021, was that vaccination in these rural areas began on this date. From among, these individuals, those presenting with SAH within 3 days after vaccination when side effects of mRNA vaccination appeared to be persistent were included,[
IRs are reported per 100,000 person-years [
Among 34,475 individuals vaccinated between March 6, 2021, and June 14, 2021, three cases presented with intracranial aneurysm rupture in the 3 days after vaccination. The IR of spontaneous SAH calculated using the person-year method with an observation period of 3 days following COVID-19 vaccination was 1058.7 per 100,000 person-years. All three individuals were women, with a median age of 63.7 years (range, 44–75 years). Two patients had histories of hypertension and dyslipidemia, while the other had no history of illness. The median interval from vaccination to onset of SAH was 2 days (range, 0–3 days). Preoperative WFNS grade was I or II in all cases. Saccular aneurysms were diagnosed using three-dimensional CT angiography or DSA in all cases. The aneurysms arose at the bifurcation of the middle cerebral artery (MCA), internal carotid-posterior communicating artery (IC-Pcom), or anterior communicating artery (Acom) in one case each. Microsurgical neck clipping was performed in two of the studied patients and aneurysmal rupture was confirmed intraoperatively. Endovascular coiling was performed in the remaining patient. Clinical outcomes at last follow-up were favorable (mRS score, 0 or 2), after a median follow-up of 57.7 days (range, 24–102 days). The demographic characteristics, clinical features, laboratory data, neuroimaging findings, and treatment course for the three patients are summarized in [
On the other hand, a total of 85 cases of ruptured aneurysms were encountered in these study areas during the same period of March 6–June 14 during the 5 years reference period before the COVID-19 pandemic (2013–2017).[
Illustrative cases
Case 1
A 44-year-old woman who had no medical history presented with severe headache 4 h after receiving the second dose of the BNT162b2 mRNA COVID-19 vaccine. On admission, she was unconscious (Glasgow coma scale [GCS] 4). Initial CT of the head revealed diffuse SAH in the basal cistern, thin subdural hematoma, intracranial hematoma (blood volume, 34.3 ml; 33 mm × 52 mm × 40 mm), and communicating hydrocephalus [
Figure 1:
Computed tomography (CT) shows subarachnoid hemorrhage (upper, left) and left Sylvian hematoma (upper, right). Preoperative CT angiography demonstrates a saccular aneurysm (white arrow) (lower, left). Intraoperative images of post clip ligation indicating rupture of the aneurysm (white arrow) (lower, right).
Case 2
A 72-year-old woman with arterial hypertension and dyslipidemia presented with severe headache 3 days after receiving the first dose of BNT162b2 mRNA COVID-19 vaccine. She was fully alert (GCS score 15) on arrival at the emergency department. Initial CT of the head revealed thin SAH in the basal cistern (Fisher II) [
Figure 2:
Computed tomography demonstrates subarachnoid hemorrhage (upper). Oblique view of the left carotid injection shows internal carotid-posterior communicating artery aneurysm with a daughter sac (white arrow) (lower, left). Postoperative angiography reveals complete obliteration of the aneurysm (white arrow) (lower, right).
Case 3
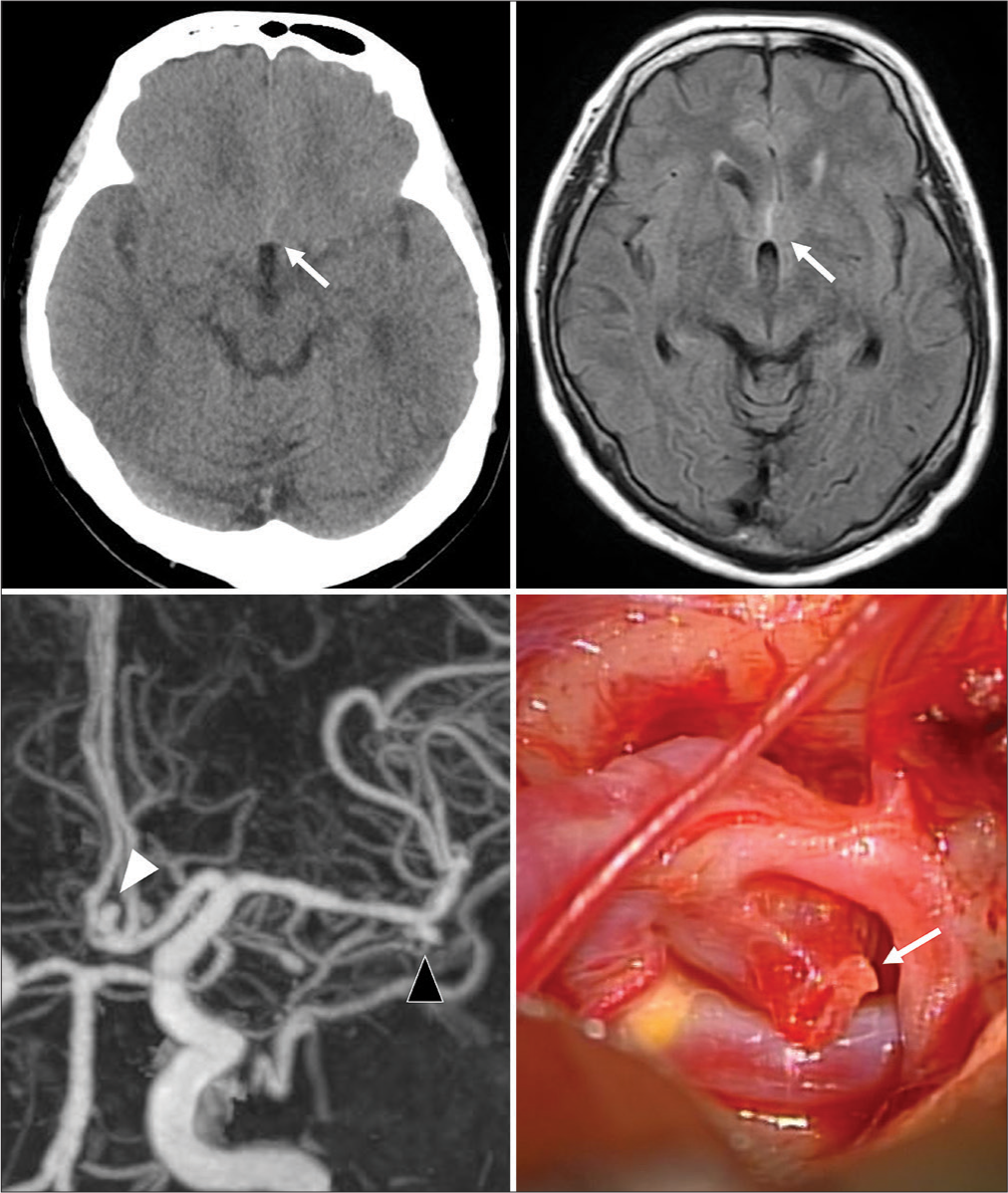
A 75-year-old woman with arterial hypertension and dyslipidemia presented with severe headache 3 days after receiving a first dose of BNT162b2 mRNA COVID-19 vaccine. She was fully alert (GCS score 15) on admission. Initial cranial CT revealed thin SAH in the interhemispheric cistern (Fisher II) [
Figure 3:
Computed tomography (CT) (upper, left) and magnetic resonance imaging (upper, right) demonstrate subarachnoid hemorrhage (white and black arrows). Aneurysms were confirmed by CT angiography (white and black arrowhead) (lower, left) and were confirmed as the bleeding source intraoperatively (white arrow) (lower, right).
DISCUSSION
We have reported here three cases of intracranial aneurysm rupture shortly after receiving the BNT162b2 mRNA COVID-19 vaccine. Three patients developed aneurysmal SAH within 3 days following vaccination in our district. Although the sample size of the present preliminary data was limited, the IR for aneurysmal SAH calculated using the person-year method with an observation period of 3 days following COVID-19 vaccination was 1058.7/100,000 person-years. On the other hand, the IR for spontaneous SAH calculated using the person-year method from 2013 to 2017 was 20.7/100,000 person-years. This IR for Iwate corresponded with an IR of 20.25/100,000 person-year in Japan.[
The mRNA contained in the Pfizer-BioNTech vaccine is translated into the viral spike protein, eliciting antibody production.[
As a limitation of this study, attention should be paid to interpret the statistical results with deeply considering the limited period, region, and number of patients. Further studies as a require to confirm the findings of the present study.
CONCLUSION
We have reported the cases of three women with intracranial aneurysm rupture shortly after undergoing BNT162b2 mRNA COVID-19 vaccination. Although we believe that the advantages of COVID-19 vaccination outweigh the risks, continuous pharmacovigilance is necessary to monitor for potentially fatal adverse events and identify any possible associations.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Kubo N.editors. The database of Iwate Stroke Registry 2021. Iwate (Japan): 2021. p.
2. Kubo N.editors. The Report of Iwate Stroke Registry 2013-2017. Iwate (Japan): 2020. p.
3. Dias L, Soares-Dos-Reis R, Meira J, Ferrao D, Soares PR, Paster A. Cerebral venous thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. J Stroke Cerebrovasc Dis. 2021. 30: 105906
4. COVID-19 Vaccine Janssen: Link between the Vaccine and the Occurrence of Thrombosis in Combination with Thrombocytopenia. Available from: https://www.ema.europa.eu/en/medicines/dhpc/covid-19-vaccine-janssen-link-between-vaccine-occurrencethrombosis-combination-thrombocytopenia [Last accessed on 2021 Nov 18].
5. Vaxzevria (Previously COVID-19 Vaccine AstraZeneca): Link between the Vaccine and the Occurrence of Thrombosis in Combination with Thrombocytopenia. Available online: https://www.ema.europa.eu/en/medicines/dhpc/vaxzevria-previouslycovid-19-vaccine-astrazeneca-link-between-vaccineoccurrencethrombosis [Last accessed on 2021 Nov 18].
6. Frösen J, Piippo A, Paetau A, Kangasniemi M, Niemela M, Hernesniemi J. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004. 35: 2287-93
7. Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T. First month of COVID-19 vaccine safety monitoring United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021. 70: 283-8
8. Gubernot D, Jazwa A, Niu M, Baumblatt J, Gee J, Moro P. US population-based background incidence rates of medical conditions for use in safety assessment of COVID-19 vaccines. Vaccine. 2021. 39: 3666-77
9. Ikawa F, Morita A, Nakayama T, Goto Y, Sakai N, Iihara K. A register-based SAH study in Japan: High incidence rate and recent decline trend based on lifestyle. J Neurosurg. 2020. 27: 1-9
10. Summary of Death Cases after the Administration of COVID-19 Vaccines. Available from: https://www.mhlw.go.jp/content/10906000/000778304.pdf [Last accessed on 2021 Oct 20].
11. Mohammad MA, Koul S, Olivecrona GK, Gӧtberg M, Tydén P, Rydberg E. Incidence and outcome of myocardial infarction treated with percutaneous coronary intervention during COVID-19 pandemic. Heart. 2020. 106: 1812-8
12. Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021. 384: 1964-5
13. Naaber P, Tserel L, Kangro K, Sepp E, Jurjensen V, Adamson A. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg Health Eur. 2021. 10: 100208
14. Omama S, Yoshida Y, Ogasawara K, Ogawa A, Ishibashi Y, Ohsawa M. Incidence rate of cerebrovascular diseases in northern Japan determined from the Iwate stroke registry with an inventory survey system. J Stroke Cerebrovasc Dis. 2013. 22: 317-22
15. Pandey AS, Gemmete JJ, Wilson TJ, Chaudhary N, Thompson BG, Morgenstern LB. High subarachnoid hemorrhage patient volume associated with lower mortality and better outcomes. Neurosurgery. 2015. 77: 462-7
16. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020. 383: 2603-15
17. Resta C Di, Ferrari D, Viganò M, Moro M, Sabetta E, Minerva M. The gender impact assessment among healthcare workers in the SARS-CoV-2 vaccination an analysis of serological response and side effects. Vaccines. 2021. 9: 522
18. Sahai H, Khurshid A.editors. Statistics in Epidemiology: Methods, Techniques, and Applications. Boca Raton: CRC Press; 1996. p. 172-4
19. See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro T. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2. S vaccination, March 2 to April 21, 2021. JAMA. 2021. 325: 2448-56
20. Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2021. 224: 108665
21. Japan gives First COVID-19 Vaccinations to Tokyo Health Workers. Available from: https://www.japantimes.co.jp/news/2021/02/17/national/vaccination-rollout-begins [Last accessed on 2021 Nov 18].
22. Yuan P, Ai P, Liu Y, Ai Z, Wang Y, Cao W. Safety, tolerability, and immunogenicity of COVID-19 vaccines: A systematic review and meta-analysis. medRxiv. 2020. 2020: 20224998