- Department of Neurology, Clinics Hospital Complex of the Federal University of Paraná, Curitiba, Brazil
- Department of Intensive care, State University of Ponta Grossa, Ponta Grossa, Brazil
- Braincare Desenvolvimento e Inovação Tecnológica SA - Brain4care, São Carlos, Brazil
- Department of Neurology, School of Medicine, University of São Paulo, São Paulo, Brazil
- Department of Clinical Analysis, State University of Ponta Grossa, Brazil
- Neurological Surgery, State University of Ponta Grossa, Ponta Grossa, Brazil.
Correspondence Address:
Caroline Link, Department of Neurology, Clinics Hospital Complex of the Federal University of Paraná, R. General Carneiro, Curitiba, Paraná, Brazil.
DOI:10.25259/SNI_314_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Caroline Link1, Thomas Markus D`Haese2, Gustavo Frigieri3, Sérgio Brasil4, José Carlos Rebuglio Vellosa5, Leonardo Welling6. Intracranial compliance and volumetry in patients with traumatic brain injury. 14-Jul-2023;14:246
How to cite this URL: Caroline Link1, Thomas Markus D`Haese2, Gustavo Frigieri3, Sérgio Brasil4, José Carlos Rebuglio Vellosa5, Leonardo Welling6. Intracranial compliance and volumetry in patients with traumatic brain injury. 14-Jul-2023;14:246. Available from: https://surgicalneurologyint.com/surgicalint-articles/12427/
Abstract
Background: Cerebral edema (CE) and intracranial hypertension (IHT) are complications of numerous neurological pathologies. However, the study of CE and noninvasive methods to predict IHT remains rudimentary. This study aims to identify in traumatic brain injury (TBI) patients the relationship between the volume of the lateral ventricles and the parameters of the noninvasive intracranial pressure waveform (nICPW).
Methods: This is an analytical, descriptive, and cross-sectional study with nonsurgical TBI patients. The monitoring of nICPW was performed with a mechanical strain gauge, and the volumetry of the lateral ventricles was calculated using the free 3D Slicer software, both during the acute phase of the injury. The linear model of fixed and random mixed effects with Gamma was used to calculate the influence of nICPW parameters (P2/P1 and time-to-peak [TTP]) values on volumetry.
Results: Considering only the fixed effects of the sample, there was P = 0.727 (95% CI [−0.653; 0.364]) for the relationship between P2/P1 and volumetry and 0.727 (95% CI [−1.657; 1.305]) for TTP and volumetry. Considering the fixed and random effects, there was P = 8.5e-10 (95% CI [−0.759; 0.355]) for the relationship between P2/P1 and volumetry and 8.5e-10 (95% CI [−2.001; 0.274]) for TTP and volumetry.
Conclusion: The present study with TBI patients found association between nICPW parameters and the volume of the lateral ventricles in the 1st days after injury.
Keywords: Computed tomography, Intracranial pressure, Traumatic brain injury
INTRODUCTION
Intracranial hypertension (IHT) is an event that occurs in different neurological pathologies, either from traumatic events, metabolic disorders, intracranial neoplasms, or cerebrovascular diseases, as cerebral ischemia or intracranial hemorrhages. Although the neurological deficits observed in patients outcomes are secondary to cerebral ischemia,[
The traumatic brain-injured (TBI) patient requires close bedside monitoring for neurological deterioration,[
Likewise, the relationship between intracranial volume and ICP is the determinants of intracranial compliance (ICC), which is possible to assess according to the dynamic changes in ICP pulse morphology or waveform (ICPW).[
MATERIALS AND METHODS
This is an analytical, descriptive, and cohort study carried out in the emergency department, wards, and ICU. The Local Ethics Committee approved this study under protocol number 3.361.003.
Patients and design
Inclusion criteria were TBI patients without craniotomy, over 18 years of age. The patients had the first monitoring of cerebral compliance within 12 h after the admission computed tomography (CT) scan and the subsequent ones, 24, 48, 72, 96, and 120 h after the CT, totaling one to six monitoring lasting 10 min each, with follow-up interrupted according to the patient’s discharge, death, or neurosurgery (the monitorization of patients submitted to decompressive craniectomy were performed only before the surgical procedure).
Neuromonitoring
CT scans were obtained on the patient’s admission and during the acute phase of the injury. Volumetry of the lateral ventricles was calculated using the free 3D Slicer software (Kitware, New York, United States). The left and right cerebral lateral ventricles were manually contoured, and based on the delineated areas, image density, and three-dimensional reconstruction, a volumetric estimation was performed by trained statisticians blinded to nICPW data.
A mechanical strain gauge (Braincare Corp. São Carlos, Brazil) subtype foil (diaphragm or SR-4) and an electronic data acquisition system with an analog-digital module connected to the sensor by an electrical wire were used for monitoring ICC. The strain gauge was applied to the temporoparietal bone, 1–1.5 cm above the ear, with engineering and technical information described elsewhere.[
Statistical analysis
The arithmetic means of the relationships found in every monitoring minute were calculated. Qualitative data were presented as n (%). Quantitative data were presented as mean + standard deviation (SD). The linear model of fixed and random mixed effects with Gamma was used to calculate the influence of P2/P1 and TTP values on volumetry. A significant P < 0.05 was considered.
RESULTS
Data sample
The data from monitoring sessions in which P2/P1, TTP, and tomography data were analyzed simultaneously, excluding data from four patients (due to insufficient P2/P1 signal acquisition quality and TTP), keeping data from a total of 50 patients.
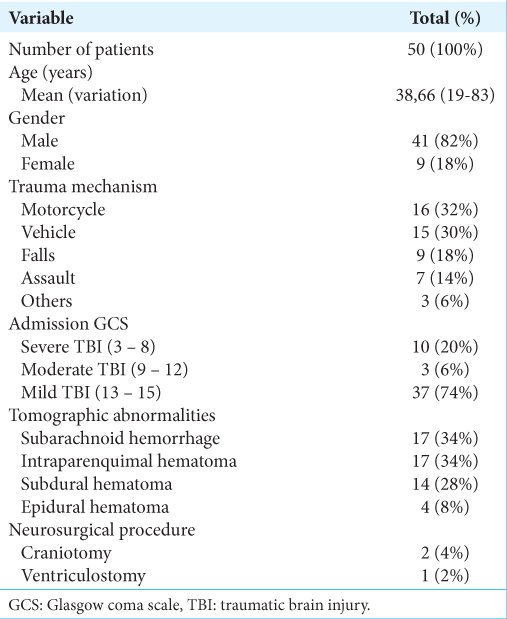
The mean age of the patients was 38.66 years (SD = 16.69; range from 19 to 83), and the male-to-female ratio was 4:1. The most common trauma mechanism was a motorcycle collision. Approximately 20% of patients had severe TBI, and the most common intracranial abnormalities were subarachnoid hemorrhage and intraparenchymal hematoma (34% each). During the monitorization, one patient presented with a small intraventricular hemorrhage without dilatation of the ventricular system and another with mild hydrocephalus and reduced brain volume. Two patients underwent decompressive craniotomy, whereas only one had external ventricular implantation and invasive ICP monitoring [
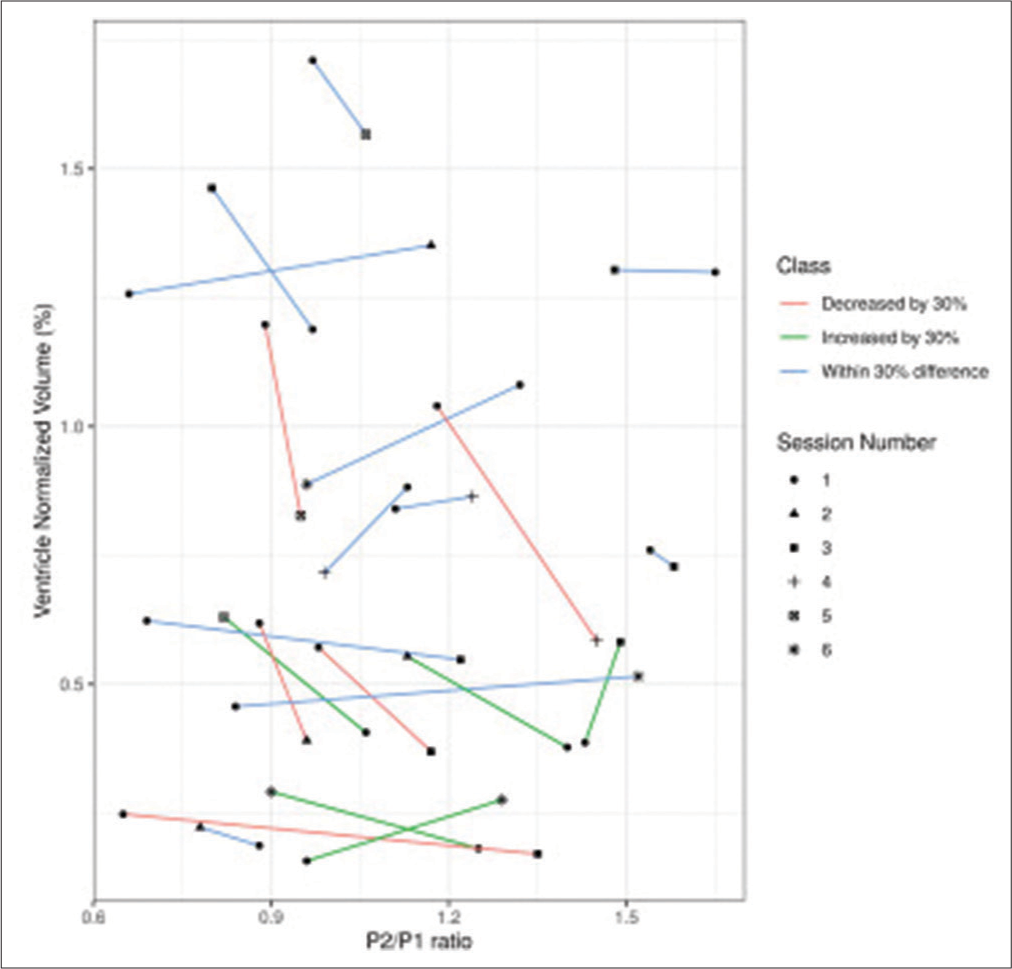
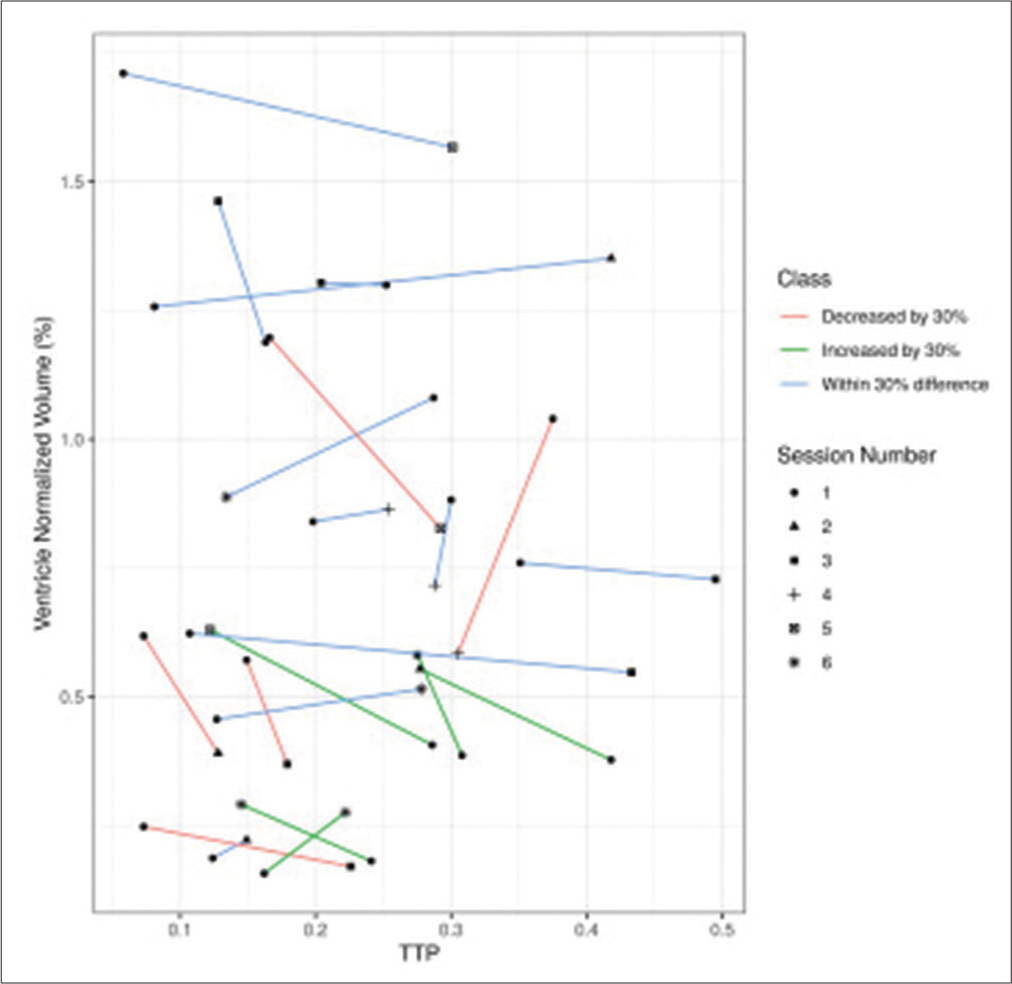
Relationship between volumetry, P2/P1, and TTP
Considering only the fixed effects of the sample (view of the entire population), there was P = 0.727 (95% confidence interval (CI) [−0.653; 0.364]) for the relationship between P2/P1 and volumetry and a P = 0.727 (95% CI [−1.657; 1.305]) for the relationship between TTP and volumetry. On the other hand, considering the fixed and random effects (individual view of each patient), there was P = 8.5e-10 (estimate = −0.181; 95% CI [−0.759; 0.355]) for the relationship between P2/P1 and volumetry and a P = 8.5e-10 (estimate = −0.685; 95% CI [−2.001; 0.274]) for the relationship between TTP and volumetry [
DISCUSSION
The Multidisciplinary Consensus Conference on Multimodal Monitoring in Neurocritical Care recently made a list of recommendations that included ICP monitoring.[
ICPw is an early marker of impaired ICC. In recent years, it has been observed as its application of new studies that analyze the ICPw and its clinical application.[
The noninvasive method used in this study does not give results in millimeters of mercury as in the invasive ICP. Instead, it shows the amplitudes of peaks P1 and P2 of the ICP pulse waveform, allowing for the assessment changes in cerebrospinal compliance.[
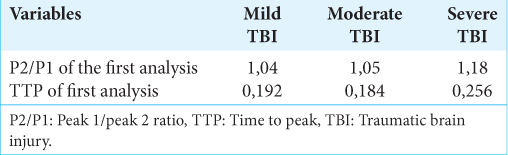
Until then, there is no literature on the relationship between the brain’s lateral ventricles volume and the variables of the intracranial pressure curve, such as the P2/P1 ratio and the TTP. There was no statistically significant difference in our study when comparing the relationship between P2/P1, TTP, and volumetry, considering only the fixed effects. However, when considering the effect of each patient, the P-value proved to be extremely significant, demonstrating that as the values of P2/P1 and TTP increase, the volume of the lateral ventricles decreases, given the effect of CE, which is common in the acute phase of the injury after TBI.
Regarding the predictive capacity of CT, specifically evaluating the tomographic classification of Marshall et al.,[
In the Marshall III classification, the cisterns are compressed but without midline deviation, and in Marshall IV, there is a deviation of the midline structures (both without intraparenchymal hemorrhages >25 cm3). It is well demonstrated that ventricular asymmetry is directly related to a worse prognosis, following the same reasoning as midline deviation. According to Toth et al.,[
Although not described in the literature, the daily neurosurgical practice demonstrates that the ventricular volume is smaller in these patients. Despite some differences in results, these studies are in agreement in demonstrating that, as changes related to the onset of CE are present, and a reduction in the volume of the ventricles is expected due to the tumefactive effect, the occurrence of increased ICP is more likely, an event related to the impairment of ICC, in which an increase in the P2/P1 and TTP ratio is expected.
In other clinical conditions, such as hydrocephalus,[
In the study by Tabaddor et al.,[
Almost all patients after a TBI have an impaired compensatory reserve. The extracranial subarachnoid space plays an important role in this mechanism by accommodating, through CSF deviation, acute variations in intracranial volume, and preventing or delaying significant increases in ICP. According to the study by Zeiler et al.,[
The indication of the pressure-volume compensation status, through the RAP, can predict the future behavior of the ICP and hemodynamic instability, containing more information than just the monitoring of the mean ICP.[
CONCLUSION
We investigated alteration in ICPW in the 1st days after TBI, comparing it with the volume of the lateral ventricles and finding an association. Bedside monitoring of intracranial parameters in a noninvasive way can offer greater ease in the therapeutic decision process, especially in contexts where the availability of invasive and expensive technologies is more restricted. Their characterization concerning noninvasive methods is relevant and already used.
The relatively more accessible cost makes this method more practicable. Its characteristic of not being invasive allows access to information in patients with less severe injuries who would not have important parameters investigated due to the absence of a formal indication.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Gustavo Frigieri declares that he has a conflict of interest with Braincare Desenvolvimento e Inovação Tecnológica SA - Brain4care, as he is a founder and Scientific Director.
Sérgio Brasil declares that he has a conflict of interest with Braincare Desenvolvimento e Inovação Tecnológica SABrain4care, as he is a scientific consultant.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
We thank our institution for the opportunity. We thank brain4care for providing the device.
References
1. Andrade RD, Oshiro HE, Miyazaki CK, Hayashi CY, Morais MA, Brunelli R. A nanometer resolution wearable wireless medical device for non invasive intracranial pressure monitoring. IEEE Sens J. 2021. 21: 22270-84
2. Ballestero MF, Frigieri G, Cabella BC, de Oliveira SM, de Oliveira RS. Prediction of intracranial hypertension through noninvasive intracranial pressure waveform analysis in pediatric hydrocephalus. Childs Nerv Syst. 2017. 33: 1517-24
3. Bigio MR. Neuropathology and structural changes in hydrocephalus. Dev Disabil Res Rev. 2009. 16: 16-22
4. Bramlett HM, Dietrich WD. Long-term consequences of traumatic brain injury: Current status of potential mechanisms of injury and neurological outcomes. J Neurotrauma. 2015. 32: 1834-48
5. Brasil S. Intracranial pressure pulse morphology: The missing link?. Intensive Care Med. 2022. 48: 1667-9
6. Brasil S, Frigieri G, Taccone FS, Robba C, Solla DJ, Nogueira RD. Noninvasive intracranial pressure waveforms for estimation of intracranial hypertension and outcome prediction in acute brain-injured patients. J Clin Monit Comput. 2022. 37: 753-60
7. Brasil S, Renck AC, Taccone FS, Solla DJ, Tomazini BM, Wayhs SY. Obesity and its implications on cerebral circulation and intracranial compliance in severe COVID-19. Obes Sci Pract. 2021. 7: 751-9
8. Brasil S, Solla DJ, Nogueira RD, Teixeira MJ, Malbouisson LM, da Silva Paiva W. A Novel noninvasive technique for intracranial pressure waveform monitoring in critical care. J Pers Med. 2021. 11: 1302
9. Brasil S, Taccone FS, Wayhs SY, Tomazini BM, Annoni F, Fonseca S. Cerebral hemodynamics and intracranial compliance impairment in critically Ill COVID-19 patients: A pilot study. Brain Sci. 2021. 11: 874
10. Bremmer R, de Jong BM, Wagemakers M, Regtien JG, van der Naalt J. The course of intracranial pressure in traumatic brain injury: Relation with outcome and CT-characteristics. Neurocrit Care. 2010. 12: 362-8
11. Calviello L, Donnelly J, Cardim D, Robba C, Zeiler FA, Smielewski P. Compensatory-reserve-weighted intracranial pressure and its association with outcome after traumatic brain injury. Neurocrit Care. 2018. 28: 212-20
12. Cardoso ER, Rowan JO, Galbraith S. Analysis of the cerebrospinal fluid pulse wave in intracranial pressure. J Neurosurg. 1983. 59: 817-21
13. Chesnut RM, Temkin N, Dikmen S, Rondina C, Videtta W, Petroni G. A method of managing severe traumatic brain injury in the absence of intracranial pressure monitoring: The imaging and clinical examination protocol. J Neurotrauma. 2018. 35: 54-63
14. Evensen KB, Eide PK. Measuring intracranial pressure by invasive, less invasive or non-invasive means: Limitations and avenues for improvement. Fluids Barriers CNS. 2020. 17: 34
15. Gmez PA, Castao-Len AM, Lora D, Cepeda S, Lagares A. Trends in computed tomography characteristics, intracranial pressure monitoring and surgical management in severe traumatic brain injury: Analysis of a data base of the past 25 years in a neurosurgery department. Neurocirugia (Astur). 2017. 28: 1-14
16. Hamilton M, Gruen JP, Luciano MG. Introduction: Adult hydrocephalus. Neurosurg Focus. 2016. 41: E1
17. Hassett CE, Uysal SP, Butler R, Moore NZ, Cardim D, Gomes JA. Assessment of cerebral autoregulation using invasive and noninvasive methods of intracranial pressure monitoring. Neurocrit Care. 2022. 38: 591-9
18. Juul N, Morris GF, Marshall SB, Marshall LF. Intracranial hypertension and cerebral perfusion pressure: Influence on neurological deterioration and outcome in severe head injury. The Executive Committee of the International Selfotel Trial. J Neurosurg. 2000. 92: 1-6
19. Kazimierska A, Kasprowicz M, Czosnyka M, Placek MM, Baledent O, Smielewski P. Compliance of the cerebrospinal space: Comparison of three methods. Acta Neurochir (Wien). 2021. 163: 1979-89
20. Lobato RD, Alen JF, Perez-Nuez A, Alday R, Gmez PA, Pascual B. Value of serial CT scanning and intracranial pressure monitoring for detecting new intracranial mass effect in severe head injury patients showing lesions Type I-II in the initial CT scan. Neurocirugia (Astur). 2005. 16: 217-34
21. Lobato RD, Gomez PA, Alday R, Rivas JJ, Dominguez J, Cabrera A. Sequential computerized tomography changes and related final outcome in severe head injury patients. Acta Neurochir (Wien). 1996. 139: 381-91
22. MacKenzie JD, Siddiqi F, Babb JS, Bagley LJ, Mannon LJ, Sinson GP. Brain atrophy in mild or moderate traumatic brain injury: A longitudinal quantitative analysis. AJNR Am J Neuroradiol. 2002. 23: 1509-15
23. Marshall LF, Marshall SB, Klauber MR, Clark MV, Eisenberg H, Jane JA. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992. 9: S287-92
24. Martin M, Lobo D, Bitot V, Couffin S, Escalard S, Mounier R. Prediction of early intracranial hypertension after severe traumatic brain injury: A prospective study. World Neurosurg. 2019. 127: e1242-8
25. Moraes FM, Rocha E, Barros FC, Freitas FG, Miranda M, Valiente RA. Waveform morphology as a surrogate for ICP monitoring: A comparison between an invasive and a noninvasive method. Neurocrit Care. 2022. 37: 219-27
26. Nucci CG, Bonis PD, Mangiola A, Santini P, Sciandrone M, Risi A. Intracranial pressure wave morphological classification: Automated analysis and clinical validation. Acta Neurochir (Wien). 2016. 158: 581-8
27. Ocamoto GN, Junior DL, Ribeiro JA, Vilela GH, Catai AM, Russo TL. Noninvasive intracranial pressure monitoring in chronic stroke patients with sedentary behavior: A pilot study. Acta Neurochir Suppl. 2021. 131: 55-8 discussion 588
28. Pineda B, Kosinski C, Kim N, Danish S, Craelius W. Assessing cerebral hemodynamic stability after brain injury. Acta Neurochir Suppl. 2017. 126: 297-301
29. Poca MA, Sahuquillo J, Matar M, Benejam B, Arikan F, Bguena M. Ventricular enlargement after moderate or severe head injury: A frequent and neglected problem. J Neurotrauma. 2005. 22: 1303-10
30. Rabelo NN, da Silva Brito J, da Silva JS, de Souza NB, Coelho G, Brasil S. The historic evolution of intracranial pressure and cerebrospinal fluid pulse pressure concepts: Two centuries of challenges. Surg Neurol Int. 2021. 12: 274
31. Robba C, Graziano F, Rebora P, Elli F, Giussani C, Oddo M. Intracranial pressure monitoring in patients with acute brain injury in the intensive care unit (SYNAPSE-ICU): An international, prospective observational cohort study. Lancet Neurol. 2021. 20: 548-58
32. Roux PL, Menon DK, Citerio G, Vespa P, Bader MK, Brophy G. The international Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: A list of recommendations and additional conclusions: A statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit Care. 2014. 21: 282-96
33. Sahuquillo J, Poca MA, Arribas M, Garnacho A, Rubio E. Interhemispheric supratentorial intracranial pressure gradients in head-injured patients: Are they clinically important?. J Neurosurg. 1999. 90: 16-26
34. Shin DS, Hwang SC, Kim BT, Jeong JH, Im SB, Shin WH. Serial brain CT scans in severe head injury without intracranial pressure monitoring. Korean J Neurotrauma. 2014. 10: 26-30
35. Tabaddor K, Danziger A, Wisoff HS. Estimation of intracranial pressure by CT scan in closed head trauma. Surg Neurol. 1982. 18: 212-5
36. Toth A, Schmalfuss I, Heaton SC, Gabrielli A, Hannay HJ, Papa L. Lateral ventricle volume asymmetry predicts midline shift in severe traumatic brain injury. J Neurotrauma. 2015. 32: 1307-11
37. Tucker B, Aston J, Dines M, Caraman E, Yacyshyn M, McCarthy M. Early brain edema is a predictor of inhospital mortality in traumatic brain injury. J Emerg Med. 2017. 53: 18-29
38. Wilkinson HA. Intracranial pressure reserve testing. Initial clinical observations. Arch Neurol. 1978. 35: 661-7
39. Zeiler FA, Kim DJ, Cabeleira M, Calviello L, Smielewski P, Czosnyka M. Impaired cerebral compensatory reserve is associated with admission imaging characteristics of diffuse insult in traumatic brain injury. Acta Neurochir (Wien). 2018. 160: 2277-87