- Department of Neurosurgery, State University of New York Upstate Medical University (SUNY), Syracuse, United States
- Department of Otolaryngology, State University of New York Upstate Medical University (SUNY), Syracuse, United States
- Department of Otolaryngology, White Plains Hospital, White Plains, New York, United States.
Correspondence Address:
Brandon Michael Wilkinson, Department of Neurosurgery, SUNY Upstate Medical University, Syracuse, New York, United States.
DOI:10.25259/SNI_931_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Brandon Michael Wilkinson1, Michael A. Duncan1, Richard Davila2, Brian Nicholas3, Harish Babu1. Intracranial malignant peripheral nerve sheath tumor: A case report and comprehensive literature review. 22-Mar-2024;15:101
How to cite this URL: Brandon Michael Wilkinson1, Michael A. Duncan1, Richard Davila2, Brian Nicholas3, Harish Babu1. Intracranial malignant peripheral nerve sheath tumor: A case report and comprehensive literature review. 22-Mar-2024;15:101. Available from: https://surgicalneurologyint.com/surgicalint-articles/12812/
Abstract
Background: Malignant peripheral nerve sheath tumors (MPNSTs) are rare malignant soft-tissue sarcomas arising from peripheral nerves. Little data exist regarding MPNST originating intracranially. Here, we present a 7th/8th nerve complex MPNST, discuss the treatment strategy and patient outcome, and provide a comprehensive review of existing literature.
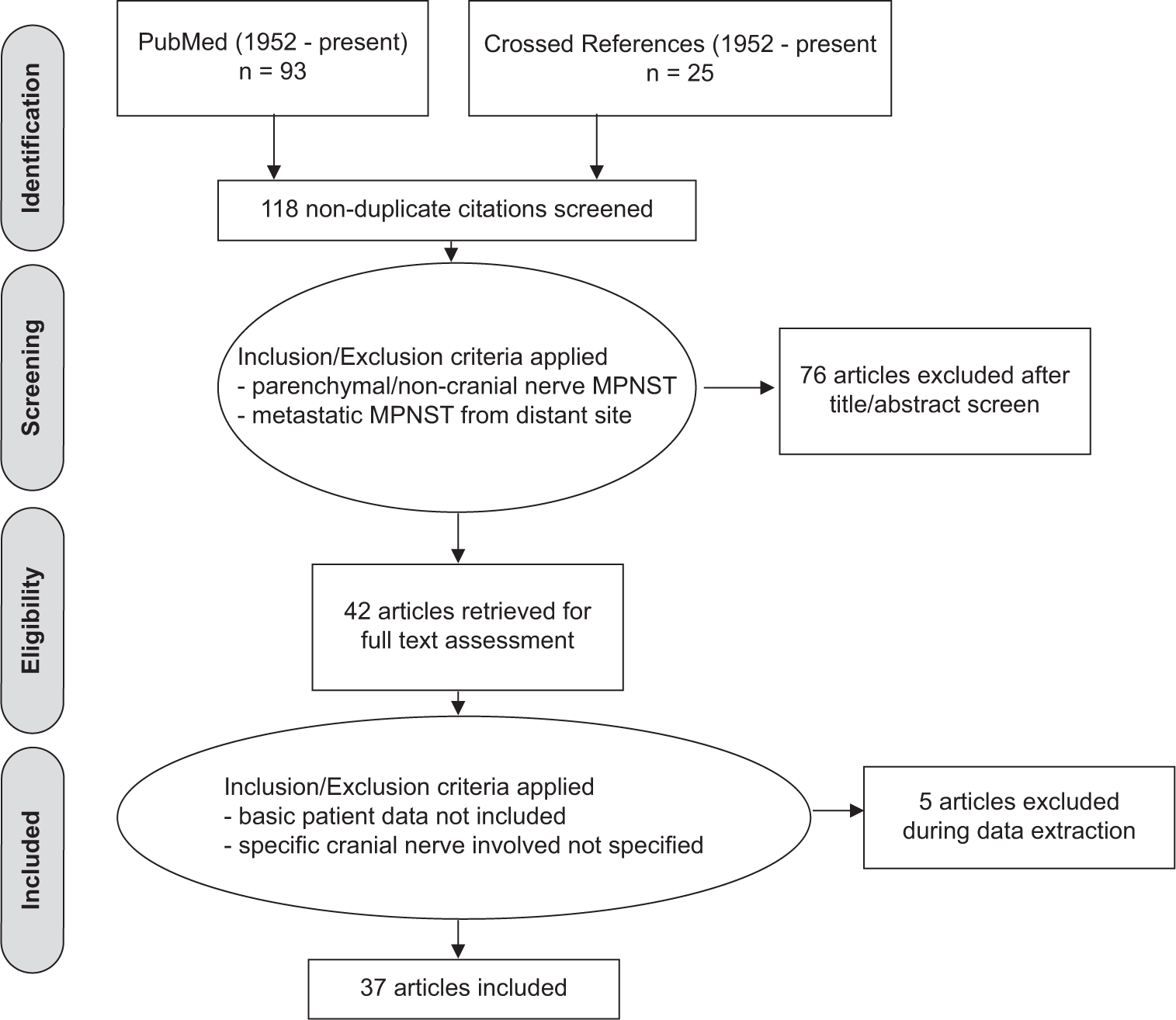
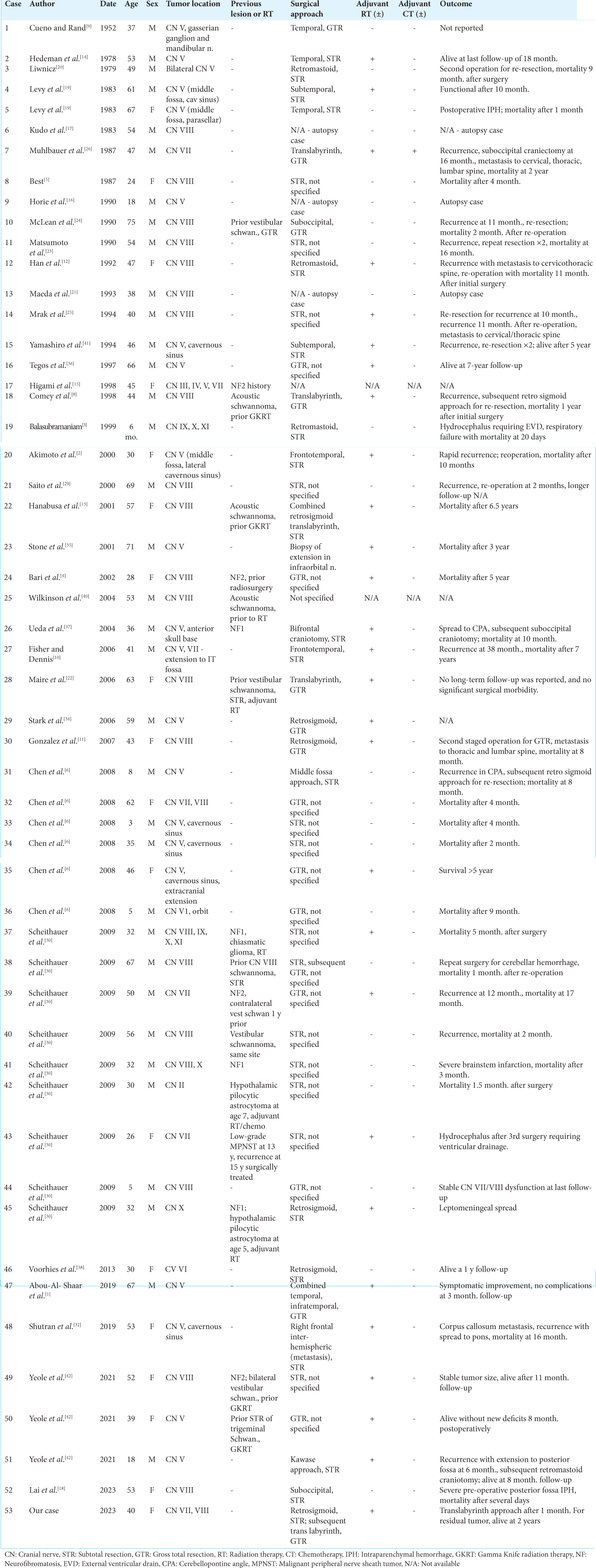
Methods: Using Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, PubMed and crossed references were queried, yielding 37 publications from 1952 to the present. Fifty-three cases of primary intracranial and extra-axial MPNST were identified.
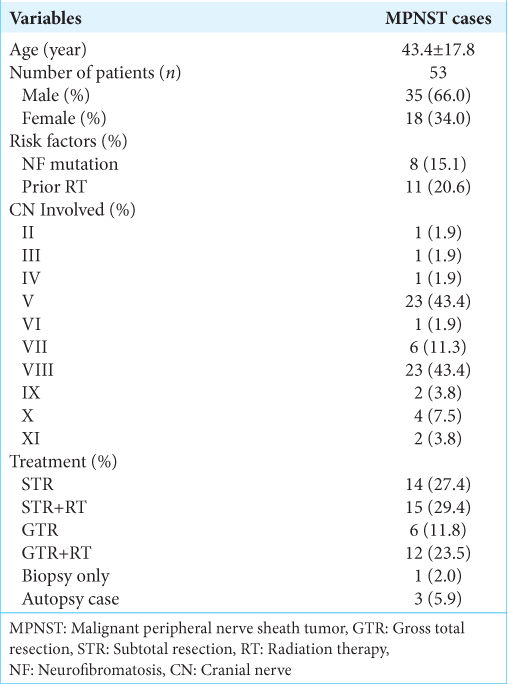
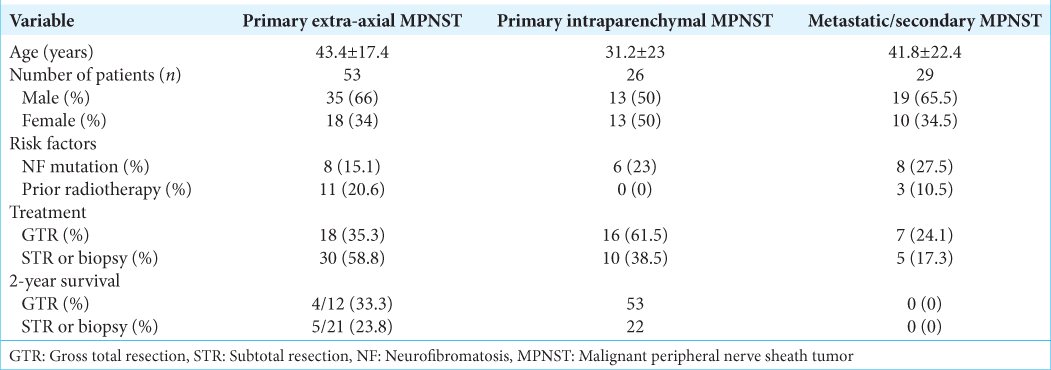
Results: We additionally report a 40-year-old female presented with acute onset dizziness and subsequent hearing loss with associated right-sided facial numbness. Magnetic resonance imaging revealed a 0.5 cm × 1.7 cm enhancing lesion within the right internal auditory canal extending into the cerebellopontine angle. The patient was initially treated with retro sigmoid craniotomy for tumor resection followed by a trans labyrinth approach for residual tumor resection. She completed adjuvant fractionated radiation therapy and underwent facial nerve transfer to restore complete hemifacial paralysis. The most common cranial nerves involved were V and VIII (43.4% each), with 66% of patients male and 34% female. The average age was 43.4 ± 17.4 years. The mean survival time for reported non-survivors after tissue diagnosis was 15 ± 4 months. Two-year survival for patients receiving gross total resection was 33.3% versus 22.8% with subtotal resection.
Conclusion: MPNSTs comprise a group of highly aggressive neoplasms that rarely arise intracranially. Gross total surgical resection should be pursued when feasible.
Keywords: Cerebellopontine angle, Intracranial, Malignant peripheral nerve sheath tumor, retro sigmoid craniotomy
INTRODUCTION
Malignant peripheral nerve sheath tumors (MPNSTs) are a rare and poorly defined heterogeneous group of malignant neoplasms comprising 5–10% of all malignant soft-tissue sarcomas.[
Intracranial MPNST is exceptionally rare, with little data guiding diagnosis and treatment strategies. Reported cases show a male predilection with age of onset predominantly in the 5th-6th decades, although widely variable. Rapid tumor growth, lesion size >5 cm, and marked heterogeneity on magnetic resonance imaging (MRI) may suggest MPNST.[
CASE REPORT
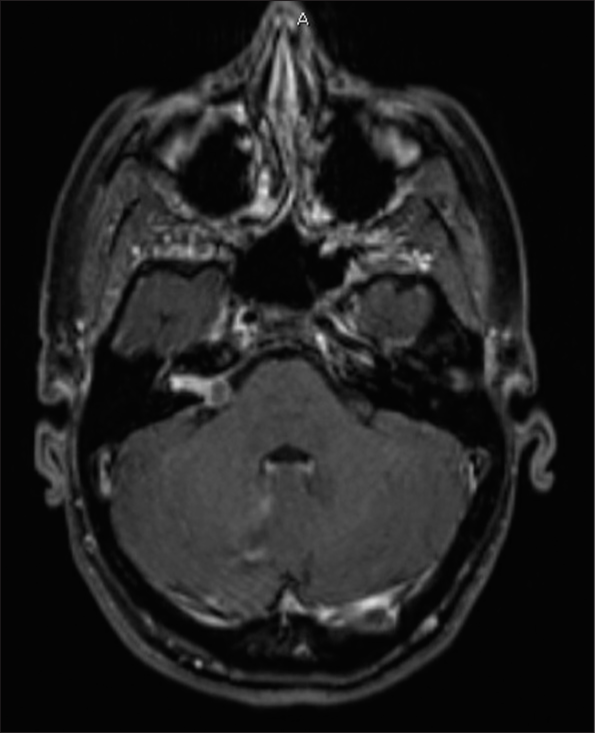
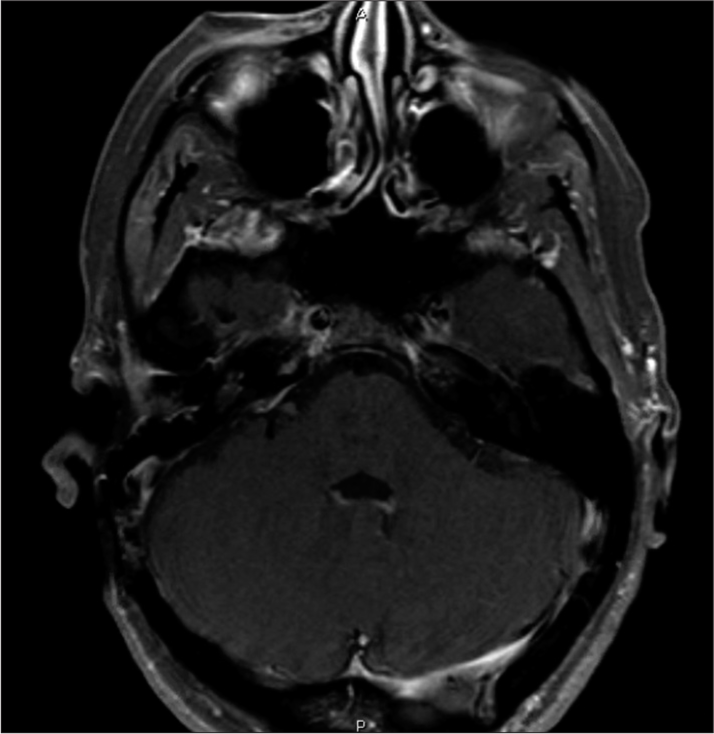
A 40-year-old female presented initially with acute onset dizziness. She later developed progressive right-sided hearing loss with the right face and tongue numbness. Initial MRI showed a 0.5 cm enhancing lesion within the right internal auditory canal (IAC). Six-month surveillance imaging demonstrated rapid tumor growth with extension into the cerebellopontine angle, measuring 1.7 cm in diameter [
On identification of the lesion, hemosiderin deposits were noted as indicative of prior hemorrhage. The tumor appeared highly vascular without clear delineation of the 7th/8th nerve complex. Frozen pathology showed numerous mitotic figures concerning malignant tumors. The decision was made to proceed with maximal total resection with 7th/8th nerve complex sacrifice. Stimulation identified the proximal nerve complex, and dissection proceeded medially to laterally. The firm tumor engulfed the nerves at the porus acusticus; thus, the petrous bone was drilled to expose the mid and lateral IAC. An additional tumor was noted laterally within the vestibule, again without clear delineation from the cranial nerves (CNs). The visible tumor mass was resected en bloc. A 45° endoscope was utilized better to visualize additional tumors within the IAC and vestibule. Further, abnormal-appearing tissue was removed without obvious evidence of gross residual tumor.
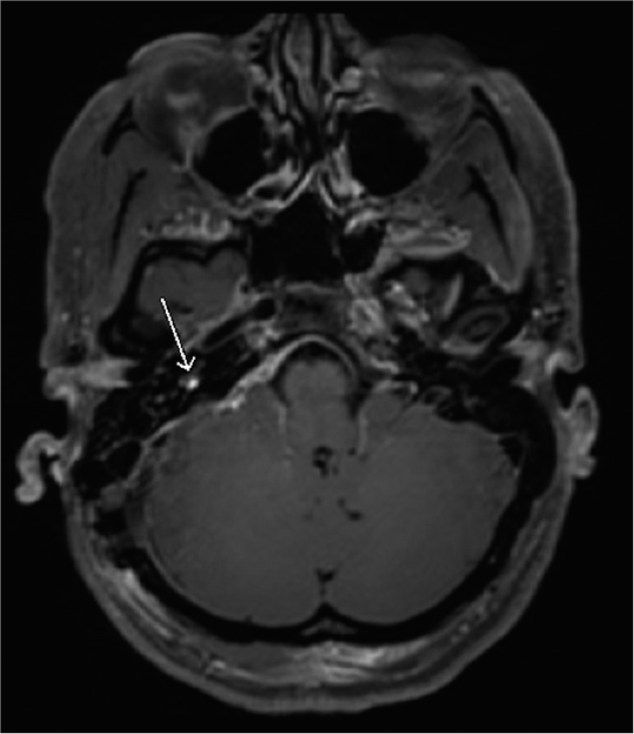
Final pathology revealed low-grade MPNST. Postoperatively, the patient had expected complete right facial paralysis (House-Brackmann VI). MRI showed a small 2 mm nodular enhancement within the vestibule concerning for residual tumor [
Her right facial weakness gradually improved, and she regained the ability to close her eyes after nine months (House-Brackmann III). Surveillance imaging two years after surgery remains unchanged. She has since returned to work and resumed her day-to-day life.
LITERATURE REVIEW
A systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. A systematic search was conducted in PubMed using the criteria “malignant peripheral nerve sheath tumor,” “intracranial,” “nerve sheath neoplasms,” and combinations of the variables “malignant,” “nerve,” “sheath,” “neurogenic sarcoma,” and “schwannoma.” The search strategy is outlined in
Inclusion criteria were as follows: (1) must be a case report, case series, or systematic review published between 1952 (first reported case of intracranial MPNST) and November 2023 when the search was performed; (2) study must report intracranial MPNST involving CN(s); (3) study must have reported patient age, sex, and specific CN involved; and (4) study must not have overlapping patients and have full-text article available through SUNY Upstate Medical University. Cases lacking information on specific CN involved, metastatic spread from distant site locations, or solely intra-axial lesions without CN involvement were excluded from the study.
Further, data extraction was performed using the following parameters: (1) author, (2) year of publication, (3) patient age and gender, (4) tumor location and specific CN involved, (5) prior surgical treatment or RT, (6) surgical approach, (7) use of adjuvant chemotherapy or radiation postoperatively, and (8) patient outcome including morbidity and mortality.
Our review yielded 37 publications from 1952 to the present describing 53 cases of intracranial MPNST originating from CNs, including our own. Patient demographic data, tumor location, treatment, and outcome are described in
DISCUSSION
MPNSTs are aggressive lesions arising from peripheral nerve or nerve sheath cells. Intracranial MPNST remains extremely rare and can be divided into two categories: extra-axial and intraparenchymal tumors. We have provided a comprehensive review of all primary intracranial extra-axial MPNST cases to date.
In addition to radiation exposure, both NF-1 and NF-2 mutations have been implicated in pathogenesis.[
Intraparenchymal, non-CN-associated MPNST has also been described. Rubino et al. reviewed 26 primary cerebral cases of MPNST arising in both supra- and infratentorial regions.[
Histopathologically, the distinction between low-grade MPNST and atypical nerve sheath tumors is challenging. Standardized grading systems for MPNST are largely lacking. Many authors use the Scheithauer et al. morphological criteria for diagnosis of low-grade MPNST, which include hypercellularity, nuclear enlargement, and hyperchromasia,[
Although surgical resection remains the mainstay of treatment, gross total resection was achieved in only 35.3% of cases, likely attributed to both technical challenges with resection and attempts to limit significant morbidity. Even with gross total resection, 2-year survival remains poor at 33.3% (vs. 23.8% with subtotal resection). Particularly in tumors involving the 7th/8th nerve complex, a thorough discussion should be had with the patient regarding potential cosmetic and functional consequences of nerve injury/sacrifice. In deciding on a retrosigmoid approach, our initial goal was to obtain a tissue diagnosis and attempt to preserve the remaining hearing function, given the small size of the tumor. Once frozen pathology suggested a malignant process, we then opted for radical dissection, which ultimately required a trans labyrinth approach. During the initial surgery, the use of a 45° endoscope assisted with the visualization of additional tumors within the IAC and may be useful in similar cases, although ultimately, a small 2 mm residual tumor remained within the vestibule.
MPNST may be indistinguishable from benign nerve sheath tumors radiographically. Tumor growth seen on short-interval surveillance imaging necessitates timely surgical evaluation and tissue diagnosis. Despite the sacrifice of the proximal 7th nerve, our patient regained significant facial function following nerve transfer. In such cases, a multidisciplinary approach should be utilized to achieve optimal patient outcomes. Two years following surgery, our patient remains without signs of disease recurrence; however, strict surveillance remains essential. Our practice is to surveillance images every three months in the first two years, then every six months after that.
CONCLUSION
MPNSTs comprise a group of highly aggressive neoplasms typically arising from peripheral nerve cells. While their intracranial involvement is rare, prompt diagnosis and treatment are essential. Gross total resection should be attempted, and a multidisciplinary approach should be utilized in similar cases where facial nerve function is compromised. Further, research on intracranial MPNST will continue to improve treatment and patient outcomes.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abou-Al-Shaar H, Gozal YM, Hunt JP, Couldwell WT. Multidisciplinary approach to malignant peripheral nerve sheath tumor of the trigeminal nerve: 2-dimensional operative video. Oper Neurosurg (Hagerstown). 2019. 17: E205
2. Akimoto J, Ito H, Kudo M. Primary intracranial malignant schwannoma of trigeminal nerve. A case report with review of the literature. Acta Neurochir (Wien). 2000. 142: 591-5
3. Balasubramaniam C. A case of malignant tumour of the jugular foramen in a young infant. Childs Nerv Syst. 1999. 15: 347-50
4. Bari ME, Forster DM, Kemeny AA, Walton L, Hardy D, Anderson JR. Malignancy in a vestibular schwannoma. Report of a case with central neurofibromatosis, treated by both stereotactic radiosurgery and surgical excision, with a review of the literature. Br J Neurosurg. 2002. 16: 284-9
5. Best PV. Malignant triton tumour in the cerebellopontine angle. Report of a case. Acta Neuropathol. 1987. 74: 92-6
6. Chen L, Mao Y, Chen H, Zhou LF. Diagnosis and management of intracranial malignant peripheral nerve sheath tumors. Neurosurgery. 2008. 62: 825-32
7. Chowdhury A, Vivanco-Suarez J, Teferi N, Belzer A, Al-Kaylani H, Challa M. Surgical management of craniospinal axis malignant peripheral nerve sheath tumors: A single-institution experience and literature review. World J Surg Oncol. 2023. 21: 338
8. Comey CH, McLaughlin MR, Jho HD, Martinez AJ, Lunsford LD. Death from a malignant cerebellopontine angle triton tumor despite stereotactic radiosurgery. Case report. J Neurosurg. 1998. 89: 653-8
9. Cueno HM, Rand CW. Tumors of the gasserian ganglion; tumor of the left gasserian ganglion associated with enlargement of the mandibular nerve; a review of the literature and case report. J Neurosurg. 1952. 9: 423-31
10. Fisher BJ, Dennis KE. Malignant epithelioid cranial nerve sheath tumor: Case report of a radiation response. J Neurooncol. 2006. 78: 173-7
11. Gonzalez LF, Lekovic GP, Eschbacher J, Coons S, Spetzler RF. A true malignant schwannoma of the eighth cranial nerve: Case report. Neurosurgery. 2007. 61: E421-2
12. Han DH, Kim DG, Chi JG, Park SH, Jung HW, Kim YG. Malignant triton tumor of the acoustic nerve. Case report. J Neurosurg. 1992. 76: 874-7
13. Hanabusa K, Morikawa A, Murata T, Taki W. Acoustic neuroma with malignant transformation. Case report. J Neurosurg. 2001. 95: 518-21
14. Hedeman LS, Lewinsky BS, Lochridge GK, Trevor R. Primary malignant schwannoma of the Gasserian ganglion. Report of two cases. J Neurosurg. 1978. 48: 279-83
15. Higami Y, Shimokawa I, Kishikawa M, Okimoto T, Ohtani H, Tomita M. Malignant peripheral nerve sheath tumors developing multifocally in the central nervous system in a patient with neurofibromatosis type 2. Clin Neuropathol. 1998. 17: 115-20
16. Horie Y, Akagi S, Taguchi K, Yoshino T, Hayashi K, Takahashi K. Malignant schwannoma arising in the intracranial trigeminal nerve. A report of an autopsy case and a review of the literature. Acta Pathol Jpn. 1990. 40: 219-25
17. Kudo M, Matsumoto M, Terao H. Malignant nerve sheath tumor of acoustic nerve. Arch Pathol Lab Med. 1983. 107: 293-7
18. Lai C, Bajin D, Chen JM, Dickson BC, Keith J, Pirouzmand F. Malignant cerebellopontine angle peripheral nerve sheath tumor with divergent mesenchymal (cartilaginous) differentiation presenting with catastrophic hemorrhage: Case report and review. J Int Adv Otol. 2023. 19: 155-8
19. Levy WJ, Ansbacher L, Byer J, Nutkiewicz A, Fratkin J. Primary malignant nerve sheath tumor of the gasserian ganglion: A report of two cases. Neurosurgery. 1983. 13: 572-6
20. Liwnicz BH. Bilateral trigeminal neurofibrosarcoma. Case report. J Neurosurg. 1979. 50: 253-6
21. Maeda M, Jozaki T, Baba S, Muro H, Shirasawa H, Ichihashi T. Malignant nerve sheath tumor with rhabdomyoblastic differentiation arising from the acoustic nerve. Acta Pathol Jpn. 1993. 43: 198-203
22. Maire JP, Huchet A, Milbeo Y, Darrouzet V, Causse N, Célérier D. Twenty years’ experience in the treatment of acoustic neuromas with fractionated radiotherapy: A review of 45 cases. Int J Radiat Oncol Biol Phys. 2006. 66: 170-8
23. Matsumoto M, Sakata Y, Sanpei K, Onagi A, Terao H, Kudo M. Malignant schwannoma of acoustic nerve: A case report. No Shinkei Geka. 1990. 18: 59-62
24. McLean CA, Laidlaw JD, Brownbill DS, Gonzales MF. Recurrence of acoustic neurilemoma as a malignant spindle-cell neoplasm. Case report. J Neurosurg. 1990. 73: 946-50
25. Mrak RE, Flanigan S, Collins CL. Malignant acoustic schwannoma. Arch Pathol Lab Med. 1994. 118: 557-61
26. Muhlbauer MS, Clark WC, Robertson JH, Gardner LG, Dohan FC. Malignant nerve sheath tumor of the facial nerve: Case report and discussion. Neurosurgery. 1987. 21: 68-73
27. Rodriguez FJ, Folpe AL, Giannini C, Perry A. Pathology of peripheral nerve sheath tumors: Diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012. 123: 295-319
28. Rubino F, Eichberg DG, Shah AH, Luther EM, Lu VM, Saad AG. When “peripheral” becomes “central”: Primary and secondary malignant intracerebral nerve sheath tumor: A case report and a systematic review. Neurosurgery. 2021. 88: 1074-87
29. Saito T, Oki S, Mikami T, Kawamoto Y, Yamaguchi S, Kuwamoto K. Malignant peripheral nerve sheath tumor with divergent cartilage differentiation from the acoustic nerve: Case report. No To Shinkei. 2000. 52: 734-9
30. Scheithauer BW, Erdogan S, Rodriguez FJ, Burger PC, Woodruff JM, Kros JM. Malignant peripheral nerve sheath tumors of cranial nerves and intracranial contents: A clinicopathologic study of 17 cases. Am J Surg Pathol. 2009. 33: 325-38
31. Scheithauer BW, Woodruff JM, Erlandson RA, editors. Tumors of the peripheral nervous system. Washington, DC: Armed Forces Institute of Pathology; 1997. p.
32. Shutran M, Mosbach D, Tataryn Z, Arkun K, Wu JK. Case report: Metastasis of a trigeminal malignant peripheral nerve sheath tumor to the corpus callosum. Neurosurgery. 2019. 84: E63-7
33. Somatilaka BN, Sadek A, McKay RM, Le LQ. Malignant peripheral nerve sheath tumor: Models, biology, and translation. Oncogene. 2022. 41: 2405-21
34. Stark AM, Buhl R, Hugo HH, Straube T, Mehdorn HM. Chronic recurrent subarachnoid hemorrhage from a trigeminal nerve malignant peripheral nerve sheath tumor: Case report. Neurosurgery. 2006. 59: E425
35. Stone JA, Cooper H, Castillo M, Mukherji SK. Malignant schwannoma of the trigeminal nerve. AJNR Am J Neuroradiol. 2001. 22: 505-7
36. Tegos S, Georgouli G, Gogos C, Polythothorakis J, Sanidas V, Mavrogiorgos C. Primary malignant schwannoma involving simultaneously the right Gasserian ganglion and the distal part of the right mandibular nerve. Case report. J Neurosurg Sci. 1997. 41: 293-7
37. Ueda R, Saito R, Horiguchi T, Nakamura Y, Ichikizaki K. Malignant peripheral nerve sheath tumor in the anterior skull base associated with neurofibromatosis type 1--case report. Neurol Med Chir (Tokyo). 2004. 44: 38-42
38. Voorhies J, Hattab EM, Cohen-Gadol AA. Malignant peripheral nerve sheath tumor of the abducens nerve and a review of the literature. World Neurosurg. 2013. 80: 654.e1-8
39. Wanebo JE, Malik JM, VandenBerg SR, Wanebo HJ, Driesen N, Persing JA. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 28 cases. Cancer. 1993. 71: 1247-53
40. Wilkinson JS, Reid H, Armstrong GR. Malignant transformation of a recurrent vestibular schwannoma. J Clin Pathol. 2004. 57: 109-10
41. Yamashiro S, Nagahiro S, Mimata C, Kuratsu J, Ushio Y. Malignant trigeminal schwannoma associated with xeroderma pigmentosum--case report. Neurol Med Chir (Tokyo). 1994. 34: 817-20
42. Yeole U, Rao KV, Beniwal M, Sivakoti S, Santosh V, Somanna S. Cranial and spinal malignant peripheral nerve sheath tumor: A pathological enigma. J Neurosci Rural Pract. 2021. 12: 770-9
43. Ziadi A, Saliba I. Malignant peripheral nerve sheath tumor of intracranial nerve: A case series review. Auris Nasus Larynx. 2010. 37: 539-45
44. Zou C, Smith KD, Liu J, Lahat G, Myers S, Wang WL. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009. 249: 1014-22