- Department of Surgery, Neurosurgery Division, University of Port Harcourt Teaching Hospital, Port Harcourt, Nigeria
- Department of Neurosurgery, Neurological Institute, Cleveland Clinic, Akron General Hospital, Cleveland, Ohio, United States
- Department of Neurosurgery, General Hospital, OstalbKlinikum Aalen, Aalen, Germany
Correspondence Address:
Uchenna Ajoku, Department of Surgery, Neurosurgery Division, University of Port Harcourt Teaching Hospital, Port Harcourt, Nigeria.
DOI:10.25259/SNI_251_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Uchenna Ajoku1, Gregory Hawryluk2, Marcel Kullmann3. Intracranial pressure monitoring and treatment practices in severe traumatic brain injury between low-and middle-income countries and high-income countries: Data or dogma?. 11-Oct-2024;15:368
How to cite this URL: Uchenna Ajoku1, Gregory Hawryluk2, Marcel Kullmann3. Intracranial pressure monitoring and treatment practices in severe traumatic brain injury between low-and middle-income countries and high-income countries: Data or dogma?. 11-Oct-2024;15:368. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13147
Abstract
Background: Traumatic brain injury (TBI) is a major cause of morbidity and mortality worldwide. Intracranial pressure (ICP) monitoring forms the cornerstone of most severe TBI (sTBI) management guidelines, yet treatment practices vary between high income countries (HIC) and low/middle-income countries (LMICs). We sought to find the reasons for variation in ICP monitoring and treatment practices between neurosurgeons in low- and high-income countries.
Methods: We developed a 34-item anonymous survey questionnaire on ICP monitoring and treatments, which was emailed to neurosurgeons of various neurosurgical societies (Africa, Asia, Europe, and North America) who manage TBI.
Results: One hundred and six respondents from 23 countries completed the questionnaire. Sixty-nine were from Africa, 16 were from North America, 12 were from Western Europe, and 8 were from Asia. About 48.72% of respondents from LMICs versus 96.43% from HICs have had training on ICP use. Among practitioners who monitor ICP invasively in
Conclusion: Significant differences exist in ICP monitoring and treatment in patients with sTBI between high and LMICs. Cost and availability are the main determinants of ICP monitor usage. Practice pattern among the respondents was not completely supported by evidence.
Keywords: Brain trauma foundation, High-income countries, pressure, Low-medium-income countries, Traumatic brain injury, Intra Cranial Pressure
INTRODUCTION
Traumatic brain injury (TBI) is a major cause of morbidity and mortality worldwide, especially among young people.[
While the evidence for recommending ICP monitoring in TBI remains shaky, variations in its application differ as one moves from one geographic region to another.[
In the absence of incontrovertible evidence, ICP treatment policies are driven by several factors. Local experiences, individual surgeon preferences, and the availability of resources are presumed determinants of practice patterns.
In this survey, we sought to find the reasons behind the variation in the practice of ICP monitoring and treatment among neurosurgeons from LMICs and high-income countries (HICs). Although ICP monitoring is considered standard of care in most HICs, among practitioners in HICs who don’t monitor ICP regularly, the reason was sort. This was compared with practice patterns among practitioners from LIMCs.
MATERIALS AND METHODS
An anonymous web-based questionnaire using Survey Monkey was developed.
The survey questions were multiple-choice questions about ICP monitoring and treatment with five main themes.
ICP training/experience Frequency/rate of ICP monitoring ICP-directed treatment thresholds Barriers or perceived barriers to monitoring Knowledge of current evidence on ICP use in TBI.
The survey questionnaires were circulated among a team of experts across four continents (Africa, Asia, Europe, and North America) who were neurosurgeons with research interests in TBI and or neurocritical care. Some of these experts have been involved in the development of various TBI guidelines. They made comments and suggestions, which resulted in the refinement of the questions to meet the study objectives. Several iterations of this process resulted in the removal of ambiguity and simplification of the subject. We then conducted a Pilot test involving 10 senior TBI experts, and their responses were used to make the final draft. An invitation to the SurveyMonkey questionnaire [
The first part of the questionnaire was on the demography of the respondents. We then asked questions about the training and experience with ICP monitoring. In this section, we explored to know what informed the use or lack of use of ICP in the management of patients with severe TBI (sTBI). The next part was on specific therapeutic interventions while using ICP monitoring and the various treatment thresholds. Finally, we sought to know how much of the respondents’ practices were supported by evidence.
Respondents were divided into five geographic regions according to where they practiced (Europe, North America, Sub-Saharan Africa, North Africa, and Indian Sub-Continent). Practitioners were classified whether they were from LMIC or HICs using World Bank International Monetary Fund classification data. We examined whether there were differences between and within geographic regions in the use of invasive ICP monitoring in TBI management. Within each region, we examined the percentage of centers where invasive ICP monitoring was part of their TBI management protocol.
Frequencies and percentages for all variables from responders were calculated using descriptive statistics. We examined factors associated with invasive ICP monitoring with the Chi-square test or Fisher’s exact test as appropriate. P < 0.05 (two-tailed) was considered statistically significant for all tests. Analyses were conducted with IBM Statistical Package for the Social Sciences for Windows release 28.
RESULTS
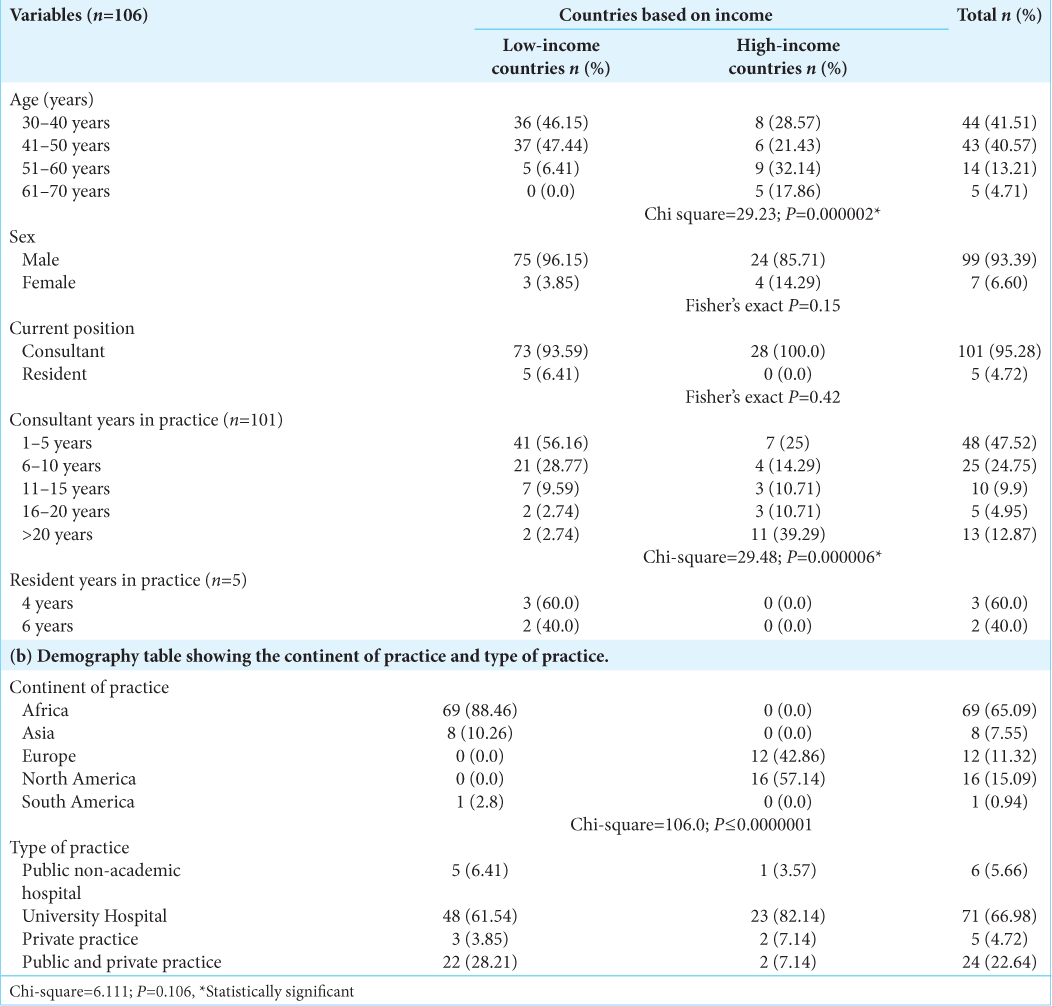
Of the 106 respondents from 23 countries who completed the questionnaire, 69 were from Africa, 16 were from North America, 12 were from Western Europe, and 8 were from Asia. The countries included Nigeria (57), USA (15), Germany (5), UK, India (4), South Africa, Egypt, Hong Kong (2), Canada, Ethiopia, Italy, Kenya, DRC, Denmark, Cote d’Ivoire, Colombia, Morocco, Malawi, Portugal, Indonesia, Sudan, and Sri Lanka (1). All but five respondents were consultants with neurotrauma practice experience ranging from 1 to >20 years. Seventy-seven of the respondents practice in LIMC, while 28 practice in HIC (World Bank Criteria)[
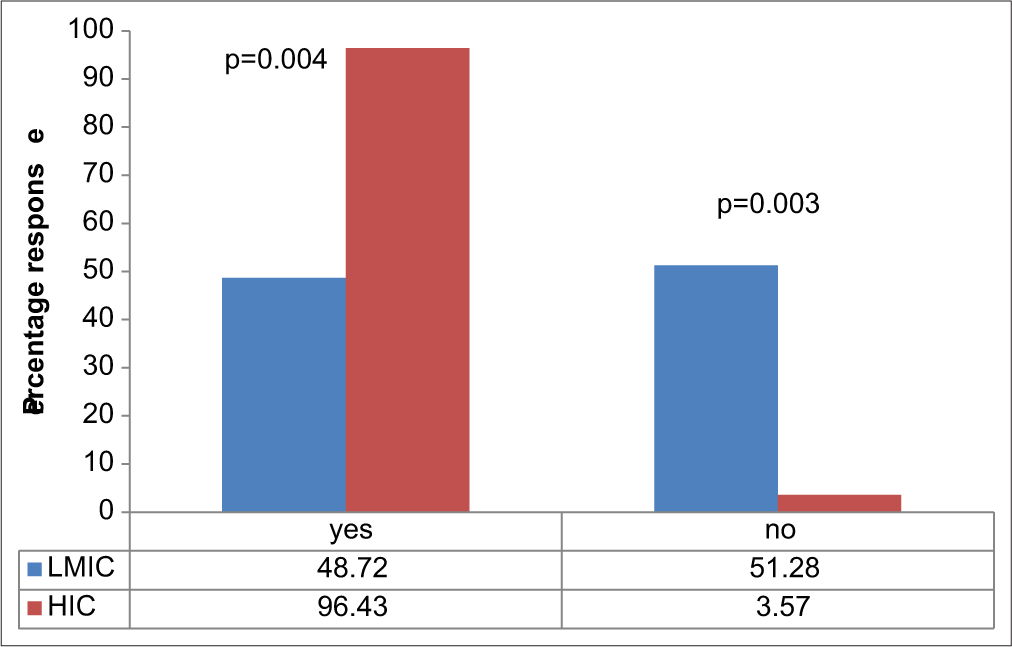
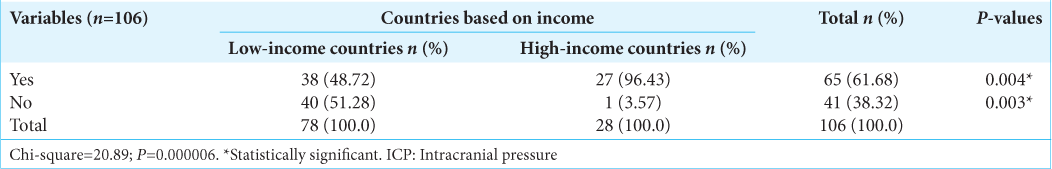
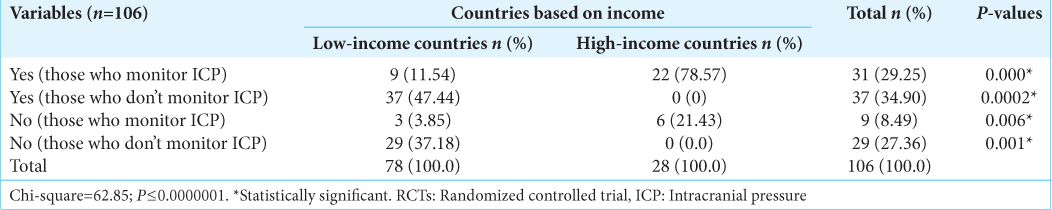
On Invasive ICP monitoring, 48.72% of respondents from LMIC versus 96.43% from HIC have had training on how to insert a probe and obtain reading (P = 0.00). About 51% of surgeons from LMIC have had no form of training on invasive ICP use/insertion, whereas all but one respondent has had this training in the HIC group [
In terms of TBI burden, 32% (25) versus 7.14% (2) (P = 0.009) of respondents from LMIC versus HIC, respectively, manage >300 cases/year. About 15 (20%) of respondents from LMIC manage more than 200 cases of sTBI versus 3 (10%) from HIC (P = 0.004) [
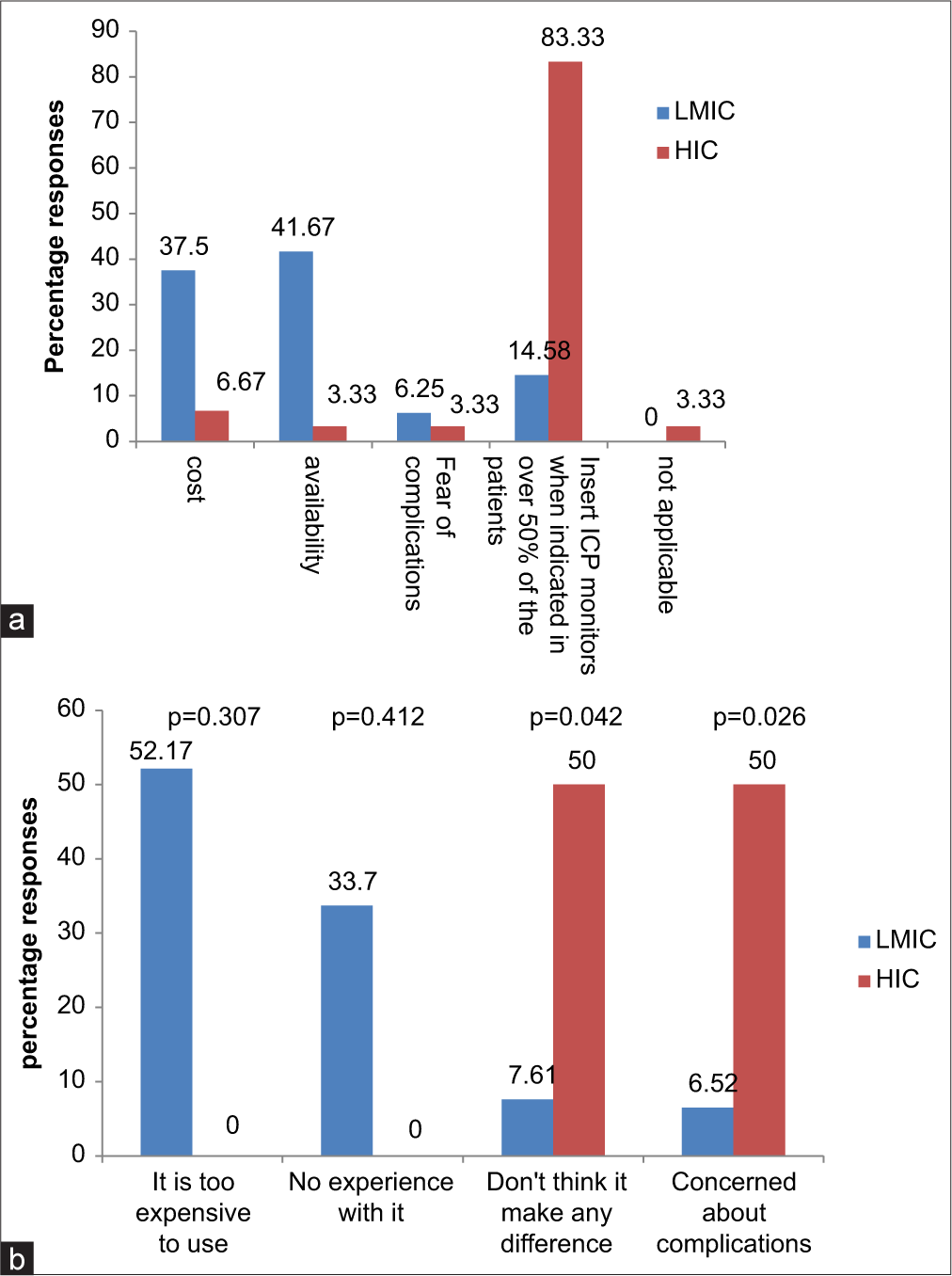
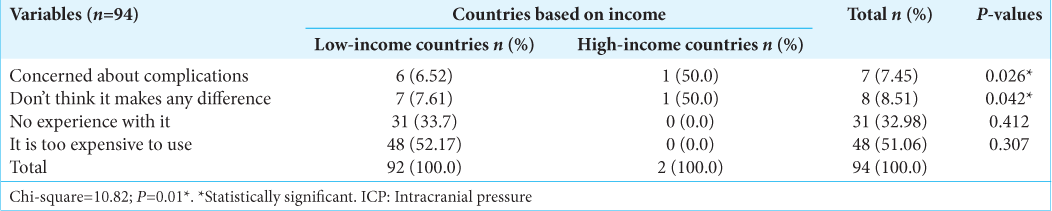
All respondents from HIC (100%) would monitor ICP invasively in patients with sTBI, whereas only 15.38% of responders in LMIC would do so (P = 0.00). Among practitioners who monitor ICP invasively but who do so in <50% of patients that need it, 41.6% from LMIC versus 3.3% from HIC (P = 0.00) cited availability of ICP monitors as the major constraints, while 37.5% from LMIC versus 3.3% from HIC said cost was the reason. Overall, the reason for not using invasive ICP monitoring among LMIC versus HIC was cost (52.17% vs. 0%), lack of experience (33.7% vs. 0%), concerns about complications (6.52% vs. 50%), and it does not make any difference (7.61% vs. 50%); P = 0.01 [
Their knowledge of randomized control trials [RCTs] on ICP monitoring [
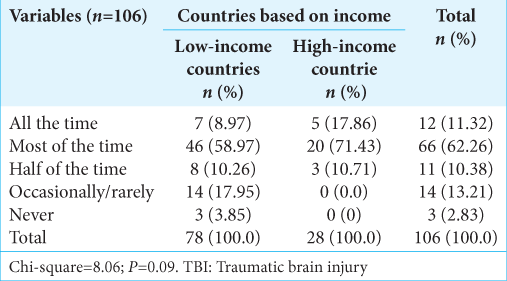
The question, which sought whether respondents think they follow the Brain Trauma Foundation (BTF) guidelines in the management of TBI, also showed a disparity between the LMIC and HIC. Only 8.97% from LMIC said that they follow BTF guidelines all the time compared to 17.86% from HIC, most of the time; 58.97% versus 71.83%, half of the time; 10.26% versus 10.71%; occasionally; 17.95% versus 0%; and never; 3.85% versus 0% (P = 0.09) [
While ICP and CPP are considered foundational in multimodality neuromonitoring in sTBI; 92.86% versus 17.95% (P = 0.00) in HIC and LMIC reported measuring them all the time. About 3.57% versus 6.41% of respondents from HIC and LMIC, respectively, monitor PBt02 all the time in patients (P = 0.00). For the pressure reactivity index, it is 7.14% versus 2.56% (P = 0.2), and for microdialysis, 3.57% versus 2.56% (P = 0.11) in HIC and LMIC, respectively.
The preferred agent of choice as the first line for control of ICP was mannitol, 27% versus 20.87% (P = 0.04) among LMIC versus HIC, respectively. Second-line ICP control measures did not differ significantly between the two groups.
DISCUSSION
The BEST TRIP Trial remains the only high-quality evidence on the impact of ICP monitoring on sTBI outcome, but the results of the trial did not show that routine monitoring makes a difference in patients.[
The BEST TRIP Trial has been criticized for lacking both internal and external validity. Nevertheless, the result of the trial should not trump common sense and basic human physiology on which ICP monitoring is predicated. Although it was a randomized controlled trial (RCT), the trial did not factor in differences in pre-hospital care or care after hospital discharge in the patient cohort. Unfortunately, it is believed that the information was not provided in the article and may have confounded the results. The trial has also been criticized for using a composite primary endpoint that had not been validated to measure TBI outcomes. The Trial also lacked external validity, according to critics, since the results could not be compared with results from HICs with advanced health-care systems.
Information obtained from invasive ICP monitoring has formed the foundation of most modern sTBI management protocols. In this survey, we found significant differences in the use of ICP monitoring and treatment of intracranial hypertension as one moves from one geographical region to another. Within a particular geographic region with similar socioeconomic status and health-care system, there was no significant difference, but as one moves across different socioeconomic regions, practice patterns change significantly [
While invasive ICP monitoring has become a routine intervention for the management of patients with sTBI in advanced healthcare systems, the level of training and adoption is significantly lower in lower-income countries. Less than half of the respondents from LMIC have had training on ICP probe placement and use in the routine clinical management of patients with sTBI, whereas almost all the respondents in HIC admitted to having been trained on ICP use and carrying out ICP monitoring when indicated.
The two main reasons for this difference in monitoring are the availability of catheters/probes (supply chain problems) and the additional cost of ICP monitoring devices, which constitute a major hindrance in most LMIC health-care settings. The average cost of an ICP probe is about $500. This is a huge amount in most developing countries where lack of universal health insurance and out-of-pocket payment makes the routine use of ICP monitoring impossible in TBI. For example, over 50% of the respondents are from Nigeria, where the monthly minimum wage is <$50! Interestingly, it is among the low socioeconomic class that the burden of TBI is highest.
Gregson et al.[
When we considered therapeutic options for ICP control, the use of mannitol as the preferred agent for first tier ICP control measure was higher in LMIC than in HIC, where hypertonic saline was the preferred agent. It is unclear why this preference exists. The efficacy of hypertonic saline and mannitol in the reduction of raised ICP has been shown in several studies.[
ICP thresholds have been a subject of debate among neurosurgeons. As an attempt to address this issue, BTF, in its 2017 edition recommended a higher threshold for the treatment of raised ICP: from 20 to 22 mmHg. To this day, physicians who manage TBI all agree that sTBI is complex and multidimensional and that a single treatment threshold for intracranial hypertension in a “one-cap-fits-all” approach is scientifically naïve. We asked what ICP threshold will necessitate non-surgical and surgical interventions. More than half of respondents from HIC will commence non-surgical ICP control measures at 20 mmHg, versus a third from LMIC. For surgical intervention, 71.43% of respondents from HIC chose an ICP threshold of 25 mmHg, while 50% from LMIC chose a threshold of 20 mmHg. While there is yet to be a consensus amongst neurosurgeons on the ICP threshold that should be attained before decompressive surgery, there was a trend toward performing surgery at a lower ICP value in LMIC. The reason for performing decompressive surgery at a lower ICP value may be related to the additional cost in patient management. Anecdotal evidence suggests that where monitoring is not routinely done due to cost, it may be reasonable to intervene earlier in such settings.
This study also highlights the controversies that exist for ICP management thresholds, a subject of several major clinical trials in the past two decades. The two largest trials to date, the DECRA, and the RESCUEicp Trials, tested the effect of surgery at two different thresholds: 20 mmHg and 25 mmHg, respectively.[
How much of contemporary practice is based on evidence? Among respondents who monitored ICP from HIC, 78.57% were aware of the existence of a RCT that investigated ICP monitoring in sTBI, and 21.43% did not know of any RCT that investigated ICP monitoring in sTBI. In the LMIC, only 11.54% of those who monitored ICP in TBI were aware of the existence of an RCT on the use of an ICP monitoring in sTBI. For physicians who monitored ICP without the knowledge of data supporting it, dogma, common sense, or institutional protocols may be responsible for their practices.
The positive impact of guideline-based management of sTBI has been demonstrated by several studies.[
The variations in ICP treatment practices in sTBI demonstrated above are not just due to differences in the socioeconomic systems in the practitioners’ country of practice, as we have demonstrated in this research. Lack of robust scientific data as well as dogma may well be contributing factors. It is our view that the present gap in scientific evidence offers a veritable opportunity for further research on ICP in sTBI.
This study has several strengths. The first is the global spread of the respondents, as well as the fact that they represent the niche of neurosurgeons who manage TBI in their various countries. Furthermore, the design of the questionnaire involved experts in TBI who have been involved in various guideline development programs at national and international levels. We also built a framework around which the study questions were formed and had this undergo several iterations before the final draft was developed.
A limitation of our study is that we had fewer than expected responses from the online circulated questionnaire. The responses from LMIC were far more than that from HIC. And even among the LMIC, the majority of the responses were from sub-Saharan Africa. Consequently, the data obtained might be skewed. In addition, this study is dependent on perceived practices rather than actual practices. Another limitation is that some of the questions were isolated and did not address specific combinations as occurs in clinical settings. For example, ICP control measures are used in several combinations, and so the response might not have reflected true life situations. Unfortunately, we did not analyze differences in the various therapeutic combinations that are often used in ICP control.
CONCLUSION
Overall, there is wide variation in ICP monitoring practices as one moves from one geographic region to another. The most significant difference is found between HIC and LMIC, underscoring the role of cost as a major driver in the adoption of ICP monitoring in sTBI. Among the HIC practitioners who do not monitor ICP, lack of convincing scientific evidence is the major reason that is being alluded to.
With the paucity of scientific evidence and the lack of capacity among practitioners in LMIC, there is a need for further scientific research as well as global collaboration to improve the overall outcome in patients with sTBI.
Ethical approval
The Institutional review board approval is not required as the study was a survey-based research.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Adeleye AO, Ogun MI. Clinical epidemiology of head injury from road-traffic trauma in a developing country in the current era. Front Neurol. 2017. 8: 695
2. Adeleye AO, Olowookere KG, Olayemi OO. Clinicoepidemiological profiles and outcomes during first hospital admission of head injury patients in Ikeja, Nigeria. A prospective cohort study. Neuroepidemiology. 2009. 32: 136-41
3. Alali AS, Fowler RA, Mainprize TG, Scales DC, Kiss A, de Mestral C. Intracranial pressure monitoring in severe traumatic brain injury: Results from the American College of Surgeons Trauma Quality Improvement Program. J Neurotrauma. 2013. 30: 1737-46
4. Ameratunga S, Hijar M, Norton R. Road-traffic injuries: Confronting disparities to address a global-health problem. Lancet. 2006. 367: 1533-40
5. Badiee S. A review of the analytical income classification. Available from: http://blogs.worldbank.org/developmenttalk/a-review-of-the-analytical-income-classification [Last accessed on 2024 Feb 26].
6. Chesnut R, Videtta W, Vespa P, Le Roux P. Intracranial pressure monitoring: Fundamental considerations and rationale for monitoring. Neurocrit Care. 2014. 21: S64-84
7. Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012. 367: 2471-81
8. Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, editors. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011. 364: 1493-502 Erratum in: N Engl J Med 2011;365:2040
9. Dawes AJ, Sacks GD, Cryer HG, Gruen JP, Preston C, Gorospe D. Compliance with evidence-based guidelines and interhospital variation in mortality for patients with severe traumatic brain injury. JAMA Surg. 2015. 150: 965-72
10. Eskandari R, Filtz MR, Davis GE, Hoesch RE. Effective treatment of refractory intracranial hypertension after traumatic brain injury with repeated boluses of 14.6% hypertonic saline. J Neurosurg. 2013. 119: 338-46
11. Faiver L, Hensler D, Rush SC, Kashlan O, Williamson CA, Rajajee V. Safety and efficacy of 23.4% sodium chloride administered via peripheral venous access for the treatment of cerebral herniation and intracranial pressure elevation. Neurocrit Care. 2021. 35: 845-52
12. Gregson BA, Rowan EN, Mitchell PM, Unterberg A, McColl EM, Chambers IR. Surgical trial in traumatic intracerebral hemorrhage (STITCH(Trauma)): Study protocol for a randomized controlled trial. Trials. 2012. 13: 193
13. , editors. Guidelines for the management of pediatric severe traumatic brain injury, third edition: Update of the brain trauma foundation guidelines. Pediatr Crit Care Med. 2019. 20: 404
14. Helbok R, Meyfroidt G, Beer R. Intracranial pressure thresholds in severe traumatic brain injury: Con: The injured brain is not aware of ICP thresholds. Intensive Care Med. 2018. 44: 1318-20
15. Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J. Trial of Decompressive Craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016. 375: 1119-30
16. Karagianni MD, Brotis AG, Gatos C, Kalamatianos T, Vrettou C, Stranjalis G. Neuromonitoring in severe traumatic brain injury: A bibliometric analysis. Neurocrit Care. 2022. 36: 1044-52
17. Kawoos U, McCarron RM, Auker CR, Chavko M. Advances in intracranial pressure monitoring and its significance in managing traumatic brain injury. Int J Mol Sci. 2015. 16: 28979-97
18. Khormi YH, Senthilselvan A, O’kelly C, Zygun D. Adherence to brain trauma foundation guidelines for intracranial pressure monitoring in severe traumatic brain injury and the effect on outcome: A population-based study. Surg Neurol Int. 2020. 11: 118
19. Melhem S, Shutter L, Kaynar A. A trial of intracranial pressure monitoring in traumatic brain injury. Crit Care. 2014. 18: 302
20. Okazaki T, Kawakita K, Kuroda Y. Hospital-level intracranial pressure monitoring utilization and functional outcome in severe traumatic brain injury: A post hoc analysis of prospective multicenter observational study. Scand J Trauma Resusc Emerg Med. 2021. 29: 5
21. Piccinini A, Lewis M, Benjamin E, Aiolfi A, Inaba K, Demetriades D. Intracranial pressure monitoring in severe traumatic brain injuries: A closer look at level 1 trauma centers in the United States. Injury. 2017. 48: 1944-50
22. Poole D, Citerio G, Helbok R, Ichai C, Meyfroidt G, Oddo M. Evidence for Mannitol as an effective agent against intracranial hypertension: An individual patient data meta-analysis. Neurocrit Care. 2020. 32: 252-61
23. Sivakumar S, Taccone FS, Rehman M, Hinson H, Naval N, Lazaridis C. Hemodynamic and neuro-monitoring for neurocritically ill patients: An international survey of intensivists. J Crit Care. 2017. 39: 40-7
24. Solagberu BA, Balogun RA, Mustafa IA, Ibrahim NA, Oludara MA, Ajani AO. Pedestrian injuries in the most densely populated city in Nigeria-an epidemic calling for control. Traffic Inj Prev. 2015. 16: 184-9
25. Stocchetti N, Penny KI, Dearden M, Braakman R, Cohadon F, Iannotti F. Intensive care management of head-injured patients in Europe: A survey from the European brain injury consortium. Intensive Care Med. 2001. 27: 400-6
26. Talving P, Karamanos E, Teixeira PG, Skiada D, Lam L, Belzberg H. Intracranial pressure monitoring in severe head injury: Compliance with Brain Trauma Foundation guidelines and effect on outcomes: A prospective study. J Neurosurg. 2013. 119: 1248-54