- Department of Neurosurgery, Brooke Army Medical Center, Roger Brooke Drive, Fort Sam Houston, San Antonio, Texas, United States,
- Department of Radiology Brooke Army Medical Center, Roger Brooke Drive, Fort Sam Houston, San Antonio, Texas, United States,
- Department of Pathology, Brooke Army Medical Center, Roger Brooke Drive, Fort Sam Houston, San Antonio, Texas, United States,
- Department of Neuropathology and Ophthalmic Pathology, The Joint Pathology Center, Silver Spring, Maryland, United States.
Correspondence Address:
Rebecca L. Dillon
Department of Neuropathology and Ophthalmic Pathology, The Joint Pathology Center, Silver Spring, Maryland, United States.
DOI:10.25259/SNI_682_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Daniel R. Klinger1, Rebecca L. Dillon2, Nathan F. Clement3, Francis J. Cloran2, Iren Horkayne-Szakaly4. Intradural extramedullary pleomorphic xanthoastrocytoma: A case report. 29-Oct-2020;11:368
How to cite this URL: Daniel R. Klinger1, Rebecca L. Dillon2, Nathan F. Clement3, Francis J. Cloran2, Iren Horkayne-Szakaly4. Intradural extramedullary pleomorphic xanthoastrocytoma: A case report. 29-Oct-2020;11:368. Available from: https://surgicalneurologyint.com/surgicalint-articles/10354/
Abstract
Background: Pleomorphic xanthoastrocytomas (PXAs) are uncommon intradural and typically intramedullary astrocytic central nervous system tumors. Although they commonly occur supratentorially, they are rarely seen in the spine.
Case Description: A 43-year-old male presented with cervical neck pain and right-sided radicular symptoms. He was found to have an intradural extramedullary mass at the C5–C6 level. The lesion was fully excised and proved to be a PXA. Of interest, the lesion did not recur on postoperative MR imaging studies obtained 7 months later.
Conclusion: While rare, primary intradural extramedullary spinal PXA has been reported. Here, we review such a lesion occurring in a 43-year-old male who did well following gross total excision of the tumor.
Keywords: Pleomorphic xanthoastrocytoma, Primary, Spinal cord tumor
INTRODUCTION
Pleomorphic xanthoastrocytomas (PXAs) are uncommon, low-grade glial neoplasms that involve the central nervous system; they account for 1% of all astrocytic tumors.[
CASE DESCRIPTION
A 43-year-old Caucasian male presented with 5 months of persistent posterior cervical neck pain radiating to the right shoulder, accompanied by paresthesias and right upper extremity radiculopathy. Although both his motor and sensory exams were intact, he was diffusely hyperreflexic (3+ bilateral biceps, brachioradialis, triceps, patella, and ankles [two beats of unsustained clonus in his right foot]) with bilateral Babinski signs. Hoffman’s signs were negative bilaterally.
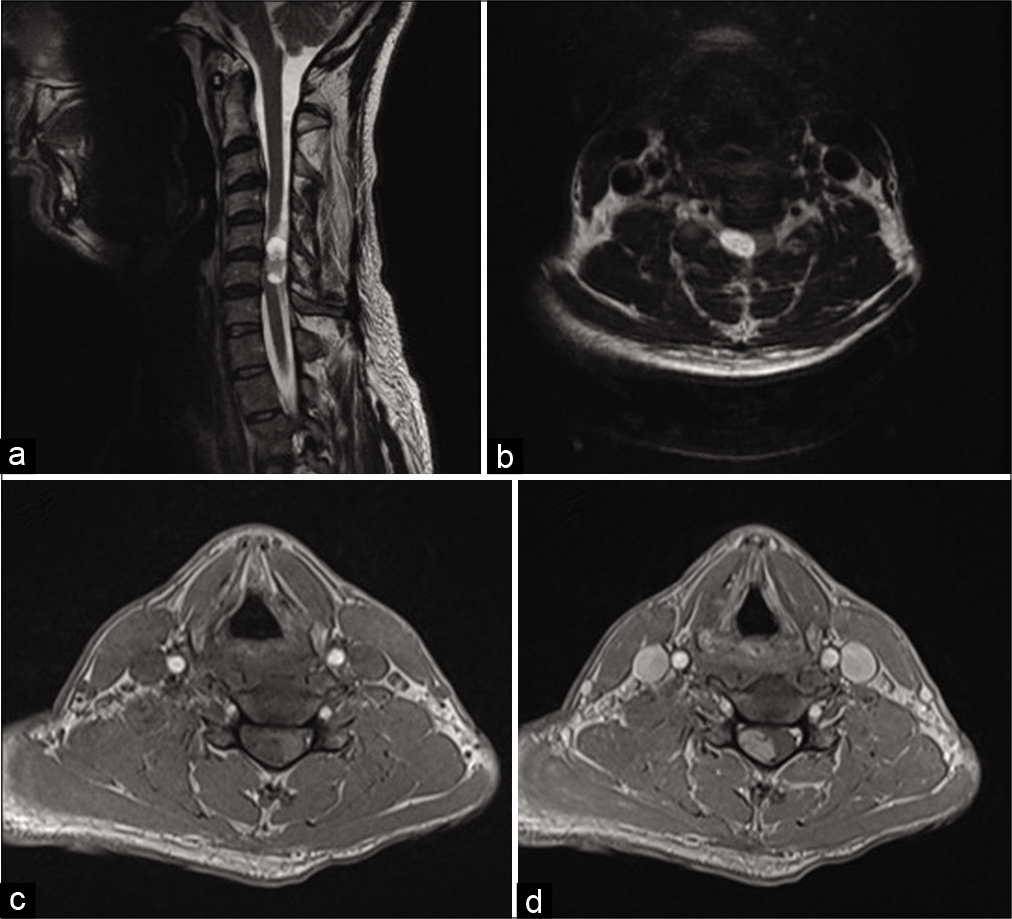
The cervical MRI demonstrated a T1 isointense, T2 hyperintense, enhancing intradural, extramedullary mass measuring 2.4 × 1.4 × 1.1 cm at the C5–C6 level [
Figure 1:
Sagittal (a) and axial T2-weighted (b) images demonstrated an intradural extramedullary cervical spine mass, displacing the spinal cord anteriorly and to the left. Axial T1 precontrast (c) and axial T1 postcontrast images (d) demonstrate heterogeneous enhancement of the mass, with a convex border abutting the spinal cord.
He underwent a cervical laminectomy for gross total microsurgical resection of the tumor. The tumor was intradural and completely extramedullary; it appeared to arise from a C6 nerve rootlet. Gross total resection of the tumor was achieved and the entire lesion for sent for pathologic analysis.
Histology
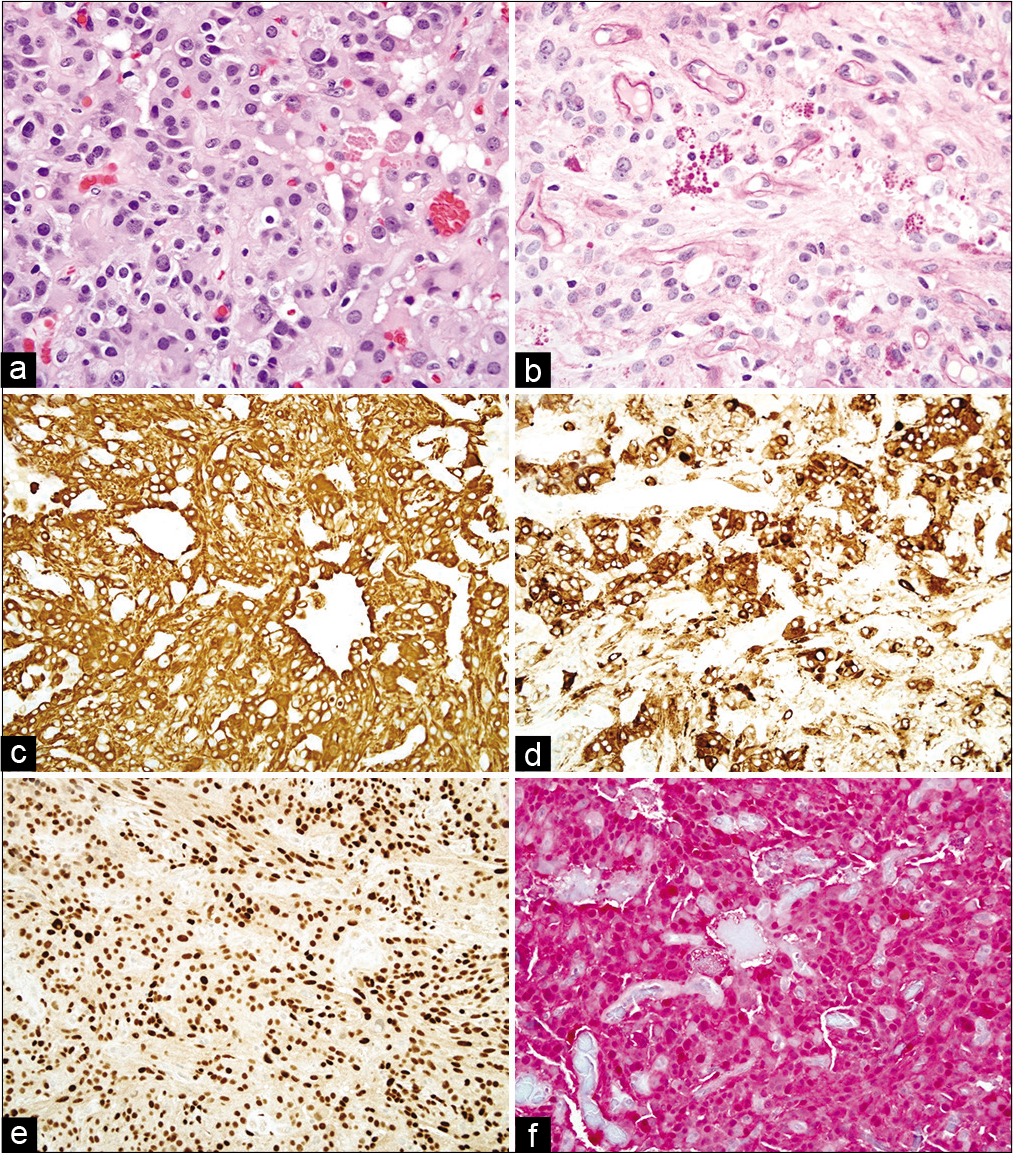
The pathologic findings were most consistent with a PXA (WHO Grade II). The tumor was a well-circumscribed neuroepithelial neoplasm arranged in fascicles, sheets, and nests [
Figure 2:
Hematoxylin and eosin stain (a) shows an astrocytic neoplasm with moderately pleomorphic nuclei and many eosinophilic granular bodies. Periodic acid–Schiff with diastase (b) highlights the eosinophilic granular bodies as well as blood vessels. By immunohistochemistry, the tumor cells show cytoplasmic positivity for GFAP (c) and synaptophysin (d), nuclear positivity for SOX10 (e), and both nuclear and cytoplasmic positivity for S-100 (f). (a,b) Viewed at ×200 magnification. (c-f) Viewed at ×400 magnification.
Postoperative course
The patient did well postoperatively, with improvement in his preoperative radicular pain and paresthesias. He was discharged home on postoperative day 2. In addition, he returned to work part-time 1 month following surgery, noting only mild right cervical paraspinal discomfort with activity.
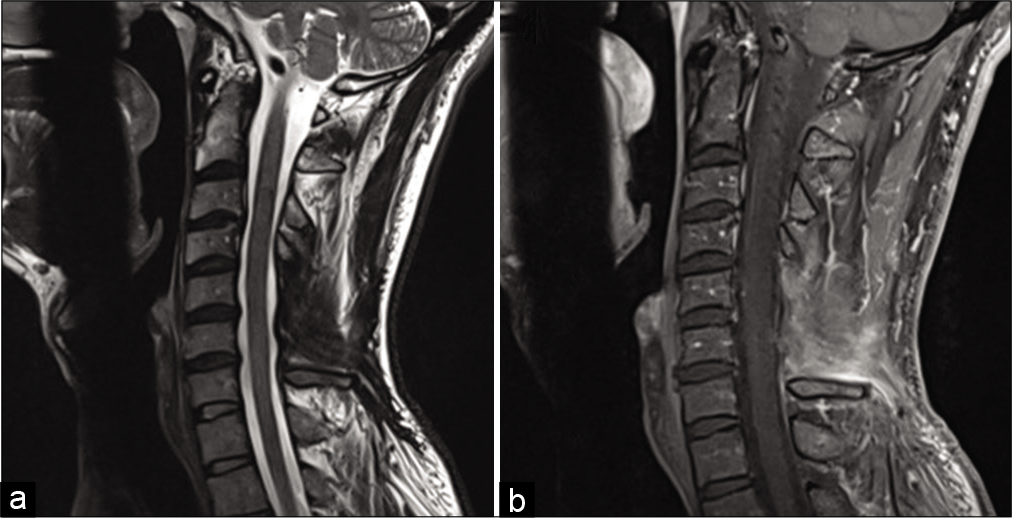
The postoperative MRI demonstrated no residual disease, and the MRI of the brain, thoracic, and lumbar spine showed no other lesions. On follow-up MR 7 months later, the tumor had not recurred [
DISCUSSION
Frequency
PXAs are rare, slowing growing central nervous system neoplasms, thought to arise from subpial astrocytes or their precursors.[
Histopathology
Histologically, these neoplasms demonstrate large pleomorphic cells, either epithelioid or spindled, which are occasionally lipidized. Multinucleated cells and eosinophilic granular bodies are frequently seen.[
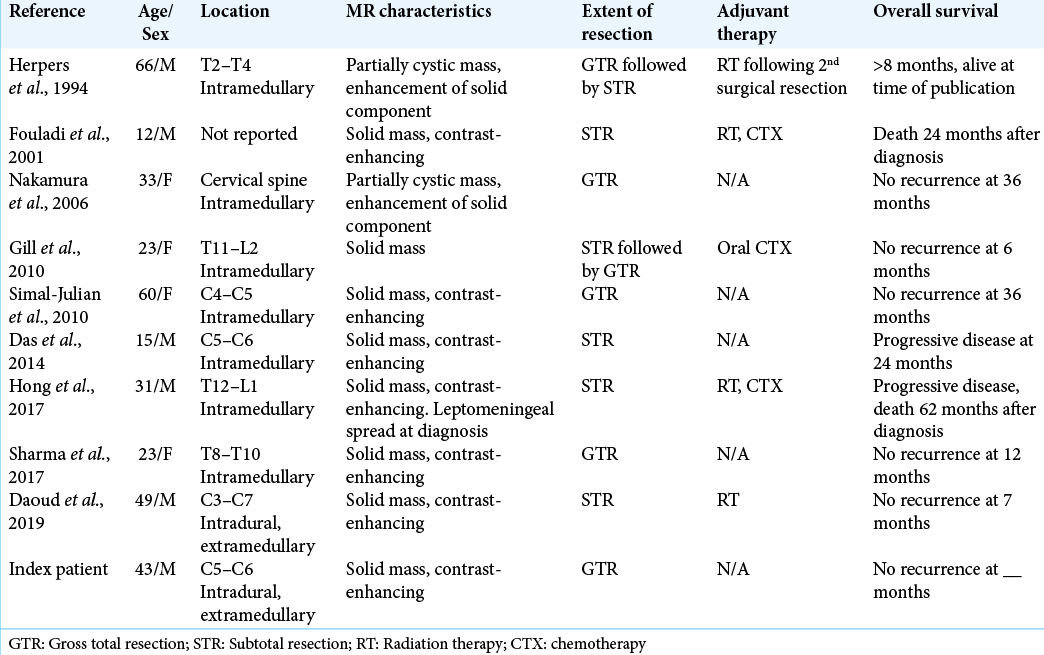
9 Prior cases reported of PXA in the spine
Only nine prior cases of primary spinal PXA have been reported; of these, only one was in an intradural, extramedullary location, the remainder being intramedullary lesions.[
Treatment protocols
There are no well-established treatment protocols for spinal PXA. Of interest, 80% of patients (4 out of 5) who underwent gross total resection were alive and without evidence of recurrent disease at time of the publication of their respective articles [
CONCLUSION
Here, we presented a patient with a primary WHO Grade II PXA involving the spinal cord in an intradural, extramedullary location. With gross total resection, the tumor has not recurred on 7-month postoperative follow-up.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abid M, Haroon S, Memon AH, Ahmad Z, Hasan SH. Pleomorphic xanthoastrocytoma; clinicopathological spectrum of an intriguing neoplasm. Pak J Med Sci. 2018. 34: 277-81
2. Benjamin C, Faustin A, Snuderl M, Benjamin DP. Anaplastic pleomorphic xanthoastrocytoma with spinal leptomeningeal spread at the time of diagnosis in an adult. J Clin Neurosci. 2015. 22: 1370-3
3. Daoud EV, Wachsmann M, Richardson TE, Mella D, Pan E, Schwarzbach A. Spinal pleomorphic xanthoastrocytoma with a QKI-RAF1 fusion. J Neuropathol Exp Neurol. 2019. 78: 10-4
4. Das S, Yip S, Hukin J, Cochrane D, Dunham C. Pleomorphic xanthoastrocytoma of the spinal cord: Case report and literature review. Clin Neuropathol. 2014. 33: 190-6
5. Dias-Santagata D, Lam Q, Vernovsky K, Vena N, Lennerz JK, Borger DR. BRAF V600E mutations are common in pleomorphic xanthoastrocytoma: Diagnostic and therapeutic implications. PLoS One. 2011. 6: e17948
6. Fouladi M, Jenkins J, Burger P, Langston J, Merchant T, Heideman R. Pleomorphic xanthoastrocytoma: Favorable outcome after complete surgical resection. Neuro Oncol. 2001. 3: 184-92
7. Gill M, Pathak HC, Madan R, Bhattacharya S, Choudhary GS. Primary spinal pleomorphic xanthoastrocytoma. Neurol India. 2010. 58: 771-3
8. Herpers MJ, Freling G, Beuls EA. Pleomorphic xanthoastrocytoma in the spinal cord. Case report. J Neurosurg. 1994. 80: 564-9
9. Hong CS, Wang JL, Dornbos D, Joehlin-Price A, Elder JB. BRAF-mutated pleomorphic xanthoastrocytoma of the spinal cord with eventual anaplastic transformation. World Neurosurg. 2017. 98: 871.e9-15
10. Kepes JJ, Kepes M, Slowik F. Fibrous xanthomas and xanthosarcomas of the meninges and the brain. Acta Neuropathol. 1973. 23: 187-99
11. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK.editors. World Health Organization, International Agency for Research on Cancer. WHO Classification of Tumours of the Central Nervous system. Lyon: International Agency for Research on Cancer; 2016. p.
12. Mallick S, Giridhar P, Benson R, Melgandi W, Rath GK. Demography, pattern of care, and survival in patients with xanthoastrocytoma: A systematic review and individual patient data analysis of 325 cases. J Neurosci Rural Pract. 2019. 10: 430-7
13. Nakamura M, Chiba K, Matsumoto M, Ikeda E. Pleomorphic xanthoastrocytoma of the spinal cord. Case report. J Neurosurg Spine. 2006. 5: 72-5
14. Okazaki T, Kageji T, Matsuzaki K, Horiguchi H, Hirose T, Watanabe H. Primary anaplastic pleomorphic xanthoastrocytoma with widespread neuroaxis dissemination at diagnosis-a pediatric case report and review of the literature. J Neurooncol. 2009. 94: 431-7
15. Pradhan P, Dey B, Srinivas BH, Jacob SE, Rathakrishnan RK. Clinico-histomorphological and immunohistochemical profile of anaplastic pleomorphic xanthoastrocytoma: Report of five cases and review of literature. Int J Hematol Oncol Stem Cell Res. 2018. 12: 265-72
16. Shaikh N, Brahmbhatt N, Kruser TJ, Kam KL, Appin CL, Wadhwani N. Pleomorphic xanthoastrocytoma: A brief review. CNS Oncol. 2019. 8: CNS39
17. Sharma M, Velho V, Binayake R, Kharosekar H. Primary pleomorphic xanthoastrocytoma of the spinal cord: A case report and review of literature. Asian J Neurosurg. 2017. 12: 566-9
18. Simal-Julian , Sanchis-Martin R, Prat-Acin R, Miranda-Lloret P, Conde-Sardon R, Cardenas-Ruiz-Valdepenas E. Spinal pleomorphic xantoastrocytoma. Case report. Neurocirugia. 2010. 21: 390-5
19. Yan J, Cheng J, Liu F, Liu X. Pleomorphic xanthoastrocytomas of adults: MRI features, molecular markers, and clinical outcomes. Sci Rep. 2018. 8: 14275
20. Zhao X, Jiang X, Wang X. Spinal pleomorphic xanthoastrocytoma companied with periventricular tumor. Int J Clin Exp Pathol. 2015. 8: 1036-40