- Department of Pediatric Neurology, Fukuoka Children’s Hospital, Fukuoka,
- Department of Neurosurgery, Fukuoka Children’s Hospital, Fukuoka,
- Department of Pediatrics, National Hospital Organization, Fukuoka-Higashi Medical Center, Koga, Fukuoka,
- Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka,
- Department of Neurosurgery, Harasanshin Hospital, Fukuoka,
- Department of Psychiatry, Shourai Hospital, Karatsu, Saga, Japan.
Correspondence Address:
Nobuya Murakami, Department of Neurosurgery, Fukuoka Children’s Hospital, Higashi-ku, Fukuoka, Japan.
DOI:10.25259/SNI_1197_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yoshie Matsubara1, Nobuya Murakami2, Ai Kurogi2, Sooyoung Lee3, Nobutaka Mukae4, Takafumi Shimogawa4, Tadahisa Shono5, Satoshi O. Suzuki6, Koji Yoshimoto4, Takato Morioka5. Intramedullary abscess at thoracolumbar region transmitted from infected dermal sinus and dermoid through retained medullary cord. 18-Feb-2022;13:54
How to cite this URL: Yoshie Matsubara1, Nobuya Murakami2, Ai Kurogi2, Sooyoung Lee3, Nobutaka Mukae4, Takafumi Shimogawa4, Tadahisa Shono5, Satoshi O. Suzuki6, Koji Yoshimoto4, Takato Morioka5. Intramedullary abscess at thoracolumbar region transmitted from infected dermal sinus and dermoid through retained medullary cord. 18-Feb-2022;13:54. Available from: https://surgicalneurologyint.com/surgicalint-articles/11405/
Abstract
Background: A retained medullary cord (RMC) is a relatively newly defined entity of closed spinal dysraphism that is thought to originate from regression failure of the medullary cord during secondary neurulation. A congenital dermal sinus (CDS) may provide a pathway for intraspinal infections such as repeated meningitis. Intramedullary abscesses are the rarest but most serious complication of a CDS.
Case Description: We treated a female infant with an intramedullary abscess in the thoracolumbar region, which was caused by infection of the CDS. Surgery revealed that the cord-like structure (C-LS) started from the cord with the intramedullary abscess, extended to the dural cul-de-sac, and further continued to the CDS tract and skin dimple. The boundary between the functional cord and the non-functional CL-S was electrophysiologically identified, and the entire length of the C-LS (the RMC) with an infected dermoid cyst was resected. As a result, the abscess cavity was opened and thorough irrigation and drainage of the pus could be performed. Histopathological examination of the C-LS revealed an infected dermoid cyst and abscess cavity with keratin debris in the fibrocollagenous tissue. The abscess cavity had a central canal-like ependymal lined lumen (CCLELL), with surrounding glial fibrillary acidic protein (GFAP)-immunopositive neuroglial tissues.
Conclusion: We demonstrated that the transmission of an infection through the RMC was involved in the development of the intramedullary abscess. A good postoperative outcome was obtained because a terminal ventriculostomy for pus drainage could be achieved by excising the nonfunctional RMC.
Keywords: Cerebrospinal fluid culture, Chemical meningitis, Ependyma, Primary neurulation, Secondary neurulation
INTRODUCTION
A retained medullary cord (RMC) is a relatively newly defined entity of closed spinal dysraphism, which is thought to originate from an almost complete arrest of apoptosis during the last phase of secondary neurulation.[
A congenital dermal sinus (CDS) consists of a tract lined by stratified squamous epithelium, which is thought to result from a focal premature disjunction between the neuroectoderm and cutaneous ectoderm during primary neurulation.[
Recently, we treated a female infant with an intramedullary abscess in the lower thoracic and lumbar region caused by an infected CDS and dermoid cysts. We demonstrated that the transmission of an infection through the cystic RMC was involved in the development of the intramedullary abscesses. Surgical strategies for these complex pathologies have also been discussed.
CASE REPORT
This female infant, who was born at 39+5 weeks of gestation without any problems during pregnancy or delivery, was found to have a dimple at the lumbosacral region at the 2-week medical checkup. At the age of 22 days, she had a fever of 39.9°C and was admitted to the local hospital. She was drowsy and irritable. Her dimples showed no signs of infection and appeared to be blind [
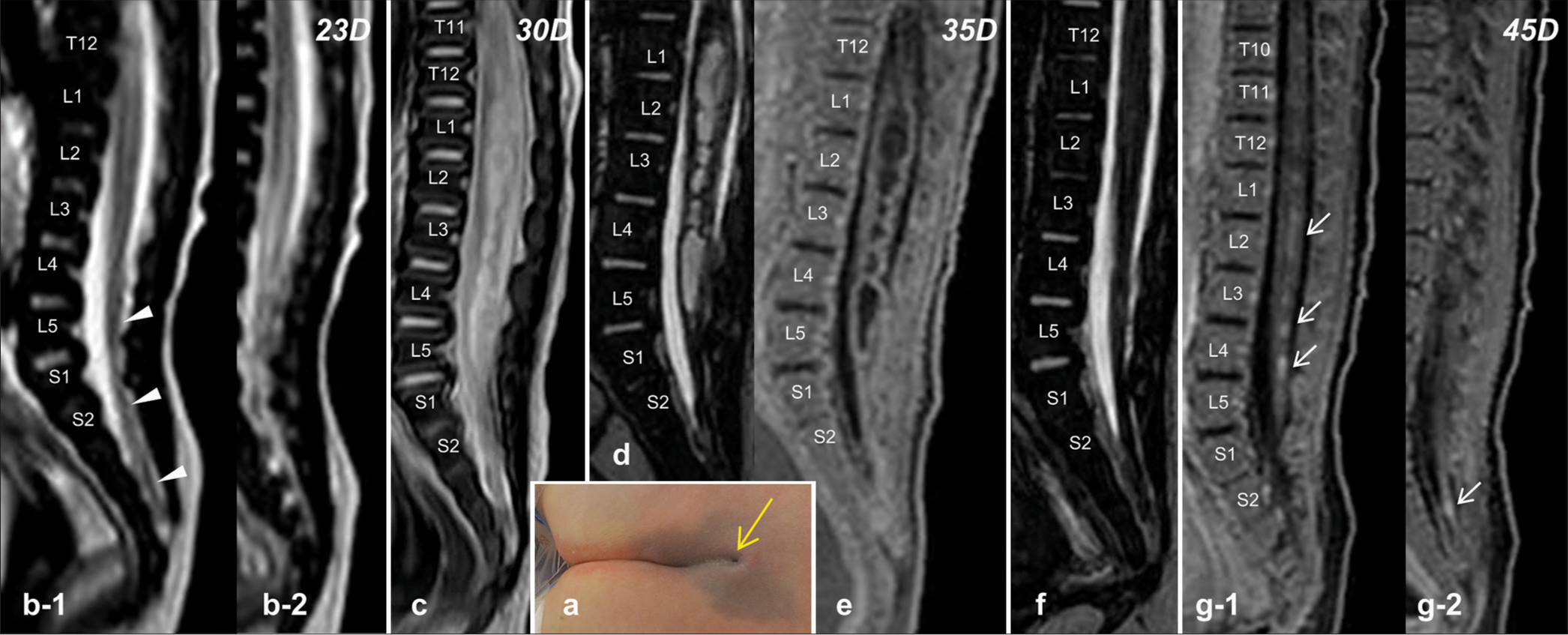
Figure 1:
(a) Photograph showing a dimple at the lumbosacral region, continuous from the gluteal cleft, which appeared to end up blind (yellow arrow). (b) Sagittal views of the T2-weighted magnetic resonance image (T2WI), performed on the 22nd day postpartum, show a cord-like structure (C-LS; arrow heads) continuous from the cord and extending to the dural cul-de-sac with spinal cord tethering, a characteristic finding for a retained medullary cord (b-1). A T2-prolonged intramedullary lesion is noted in the lower thoracic and lumbar cord (b-2). (c) T2WI on the 30th day demonstrates exacerbation of the intramedullary lesions. (d) Three-dimensional heavily T2WI image (3D-hT2WI) on the 35th day shows persistence of the intramedullary lesion with hydromyelia-like changes. (e) T1-weighed image with fat suppression (T1WI) fails to reveal the enhancing effect of the hydromyelic lesion following administration of gadolinium contrast medium (Gd). (f) 3D-hT2WI on the 45th day shows reduction of hydromyelic lesions. (g) T1WIs with the administration of Gd reveal the enhancing effect of the hydromyelic lesion (arrows, g-1) and distal part of the C-LS (arrow, g-2).
On the 25th day, her fever disappeared and her general condition improved. The number of CSF cells decreased to 1190 mm3. However, on the 28th day, she suddenly had urinary retention and a urethral catheter was introduced. MRI performed on the 30th day showed an exacerbation of intramedullary lesions [
CSF examination on the 45th day showed a decrease of 42 mm3 in the cell number and 121 mg/dL in the protein level. MRI showed a reduction of hydromyelic lesions [
At the age of 55 days, semi-emergent surgery was performed. The dimple did not end at the blind end, and when the keratin-like substance at the bottom was excised, it continued to the deep sinus. The CDS tract began at the dimple and went through the myofascial defect below the level of the bifid S3. Laminoplastic laminotomy of L3-L5 and the dura opening revealed that the swollen C-LS, which partly appeared whitish on the surface, started from the cord, extended to the dural cul-de-sac, and continued to the epidural CDS tract and skin lesion [
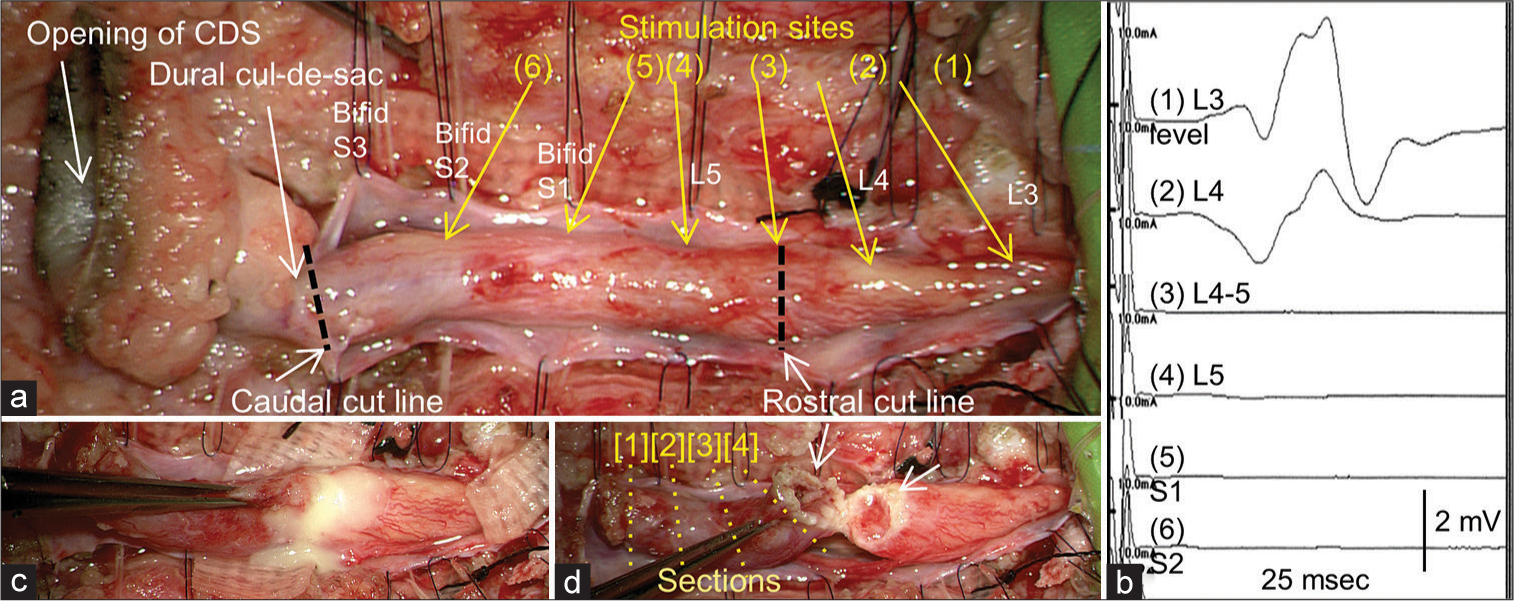
Figure 2:
(a, c, and d) Microscopic view of the operative findings and (b) intraoperative neurophysiological monitoring (IONM). (a) Laminoplastic laminotomy of L3-L5 and the dura opening reveals that the swollen cord-like structure (C-LS) starts from the cord, extends to the dural cul-de-sac, and further continues to the epidural tract and skin lesion. The border between the cord and C-LS is determined at the L4-5 vertebral level with IONM by tracing the evoked compound muscle action potentials of the gastrocnemius with stimulation, beginning from the functional cord (a, b (1) and (2)) and proceeding to the nonfunctional C-LS (a, b (3), (4), (5), and (6)). (c) A rostral incision is made on the dorsal surface of the neurophysiological border between the cord and C-LS, and pus flows out from the intramedullary abscess. (d) After irrigation of the pus in the opened abscess cavity (white arrows), the C-LS is resected as a column. The numbers [1], [2], [3], and [4] indicate the position of the section of the resected nonfunctional retained medullary cord in Figure 3.
Postoperatively, no de novo neurological abnormalities were observed and her fever reduced. Histopathologically, the epidural tract continuous from the dimple had a CDS lined by stratified squamous epithelium and contained keratin debris in the fibrocollagenous tract. The column of the resected C-LS was divided into four axial sections and, designated, from the caudal to the rostral sides, as sections [1], [2], [3], and [4], respectively [
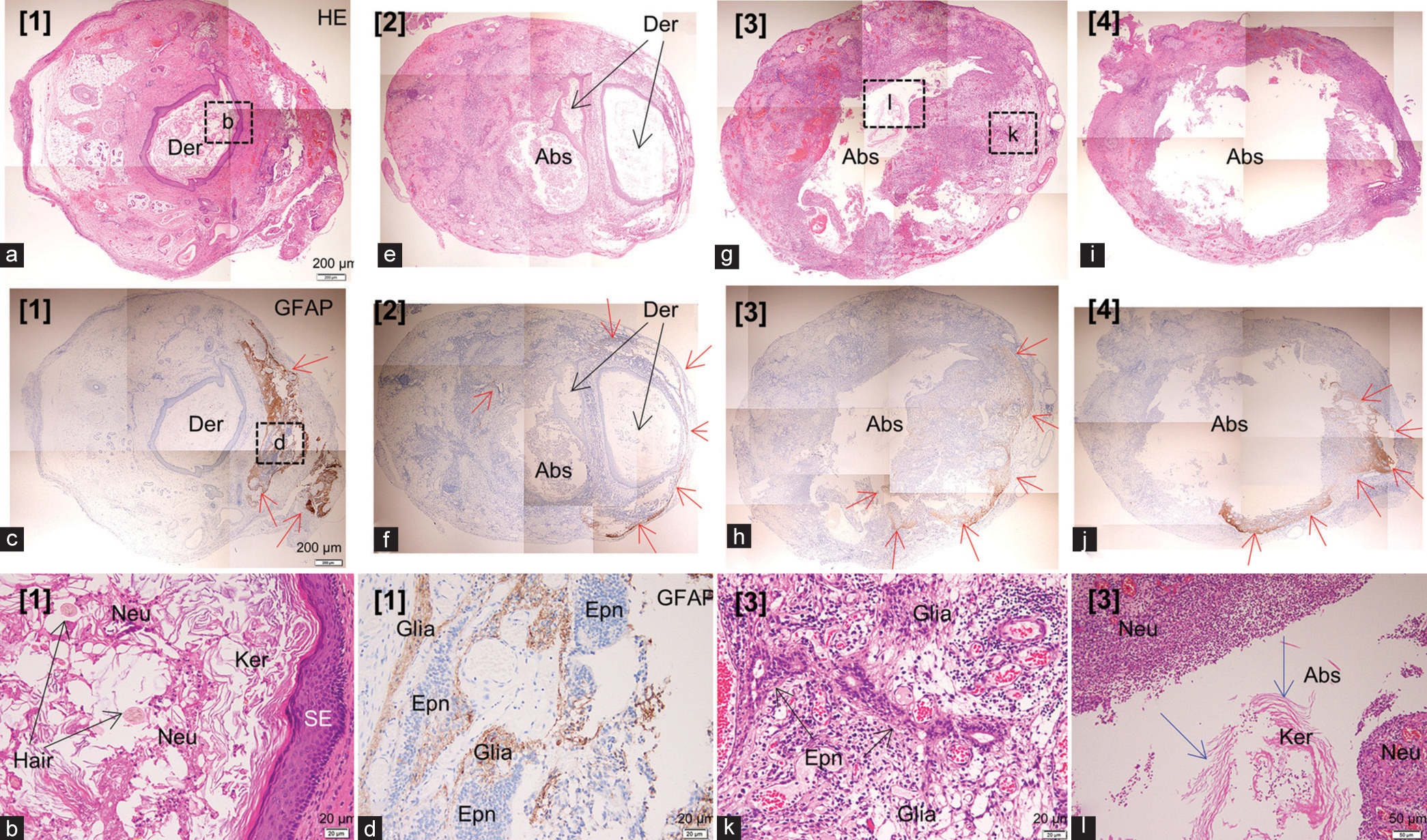
Figure 3:
Histopathological findings of the resected cord-like structure (C-LS). The column of the C-LS is divided into four axial sections and designated, from the caudal to the rostral sides, as sections [1], [2], [3], and [4], respectively, as shown in Figure 2d (a). (c), (e) and (f), (g) and (h), and (i) and (j) are the lower magnification views of the sections [1], [2], [3], and [4], respectively, stained with hematoxylin and eosin (a, e, g, and i) and glial fibrillary acidic protein (GFAP)-immunostaining (c, f, h, and j), respectively. (b, d, k, and l) Higher magnification views of the area are indicated by the dashed squares in a, c, and g. (a-d). Section [1] consists of a dermoid cyst (Der), which is lined by squamous epithelium and contains numerous neutrophils (Neu), in addition to keratin debris (Ker) and hair shafts (Hair), and GFAP-immunopositive neuroglial tissues (Glia, red arrows) with a small central canal-like ependymal (Epn)-lined lumen (CC-LELL) in the fibrocollagenous tissue (e, f). Section [2] has both the dermoid cyst and abscess cavity (Abs) in the fibrocollagenous tissue. GFAP-immunopositive neuroglial tissues are present at the periphery of the C-LS (red arrows) (g-l). On sections [3] and [4], a large abscess cavity, partially surrounded by GFAPimmunopositive neuroglial tissues (red arrows) with a small CC-LELL, is noted, while there is no squamous epithelium component. In the abscess cavity, keratin debris is observed (l, blue arrows).
Bacterial culture of the pus confirmed that the causative agents were Streptococcus anginosus, Prevotella bivia, and Enterococcus faecalis, and antibiotics with meropenem and linezolid were administered for 6 weeks postoperatively. MRI performed on the 43rd postoperative day demonstrated that the hydromyelic lesion and intramedullary abscess disappeared and untethering of the cord were achieved [
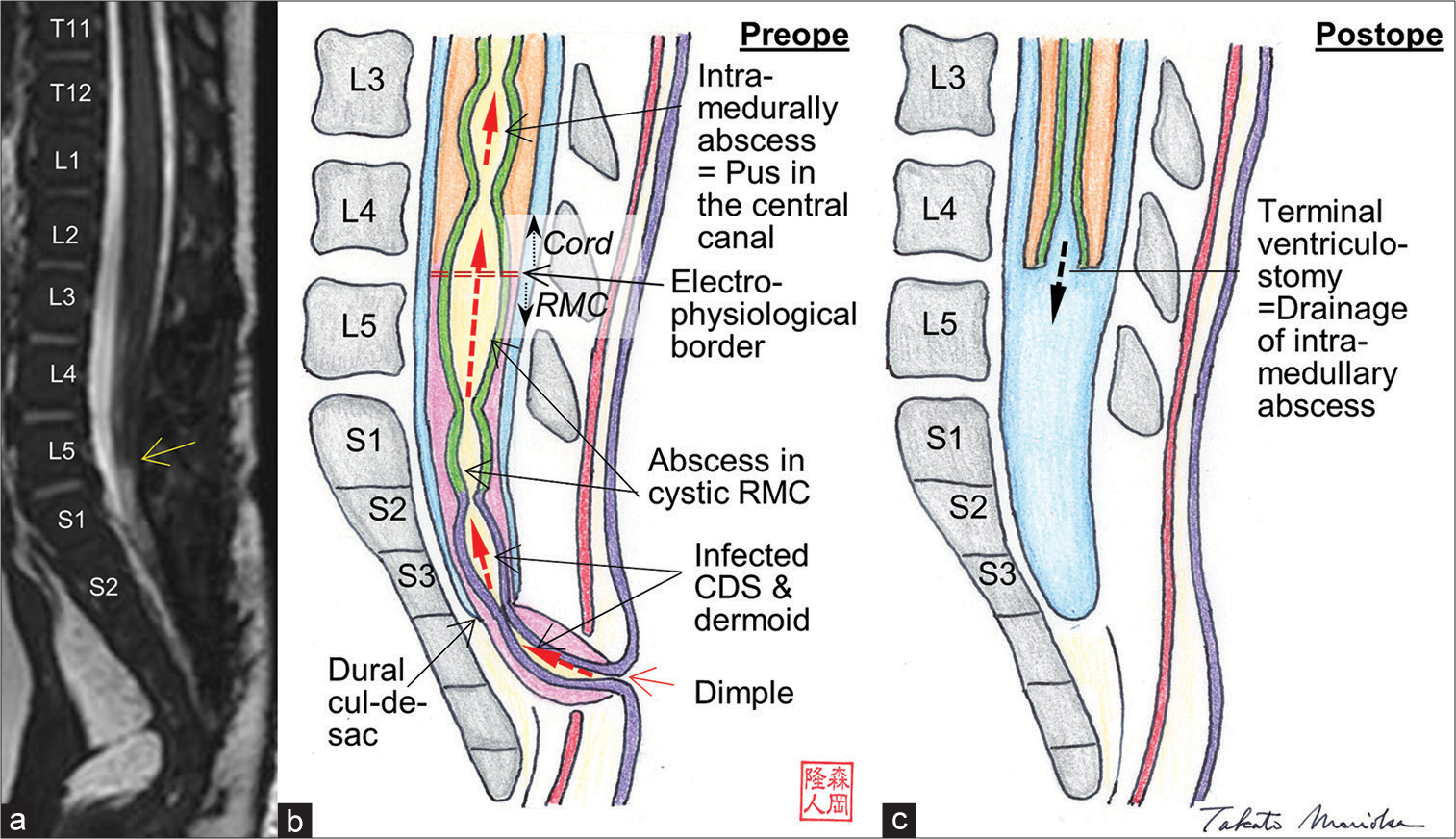
Figure 4:
(a) 3D-hT2WI on the 43rd postoperative day demonstrates that the hydromyelic lesion and intramedullary abscess had disappeared, and untethering of the cord was achieved. The most caudal position of the cord is located at the L5-S1 vertebral level (yellow arrow) (b and c). Schematic drawing of the preoperative (b) and postoperative (c) pathophysiological states of this patient. All congenital dermal sinus tracts and cords are shown as larger than the actual size to more clearly demonstrate the pathological findings. Squamous epithelial and ependymal linings are demonstrated as purple and green, respectively. The ependymal lining in the cystic retained medullary cord was not verified but described in this figure. Red dot arrows indicate the transmission of infection. See details in the text.
DISCUSSION
In the present case, the C-LS was neuroradiologically, electrophysiologically, and histopathologically consistent with RMC. Detailed histopathological findings of the C-LS showed GFAP-immunopositive neuroglial tissues with CCLELL in all four sections and a dermoid cyst in two sections of the caudal side, indicating that the CDS and the dermoid cyst were continuous from the skin dimple and extended to the caudal RMC. In addition, there were many neutrophils in the caudal dermoid cyst and keratin debris in the rostral abscess cavity. The abscess in the RMC was originally thought to be a cystic dilatation of CC-LELL, because part of the wall was surrounded by GFAP-immunopositive neuroglial tissues with CC-LELL. Normally, the wall of the cystic RMC would be lined by ependymal cells, but it is possible that they were lost during abscess formation. These findings indicate that the bacterial infection from the CDS was transmitted first to the intradural dermoid at the caudal side of the RMC, then to the cystic RMC, and finally to the continuous central canal of the thoracolumbar cord, forming an intramedullary abscess [
It is pathoembryologically unlikely that CDS of primary neurulation failure is associated with RMC, which is considered to be a secondary neurulation failure, at the same site; however, we have reported two similar cases.[
Intramedullary abscesses often leave permanent and severe neurological sequelae and require early surgical intervention. [
Surgical treatments for intramedullary abscesses associated with CDS and dermoid cysts include complete removal of the CDS tract, which is the route of infection and drainage of the abscess.[
CONCLUSION
We demonstrated that the transmission of an infection via the RMC was involved in the development of the intramedullary abscess. A good postoperative outcome was obtained because a terminal ventriculostomy for pus drainage could be achieved by excising the non-functional RMC.
Ethics statement
The authors confirm that written informed consent was obtained from the family of the infant described in this report. The authors declare that this work complies with the guidelines for human studies, and the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This work was partly supported by the Research Foundation of Fukuoka Children’ Hospital.
Conflicts of interest
There are no conflicts of interest.
References
1. Al Barbarawi M, Khriesat W, Qudsieh S, Qudsich H, Loai AA. Management of intramedullary spinal cord abscess: Experience with four cases, pathophysiology and outcomes. Eur Spine J. 2009. 18: 710-7
2. Karaaslan B, Ülkü G, Ucar M, Demirdaĝ TB, Ínan A, Börcek AÖ. Intramedullary dermoid cyst infection mimicking holocord tumor: Should radical resection be mandatory? A case report. Childs Nerv Syst. 2016. 32: 2249-53
3. Kim JW, Wang KC, Chong S, Kim SK, Lee JY. Limited dorsal myeloschisis: Reconsideration of its embryo origin. Neurosurgery. 2020. 86: 93-100
4. Kim KH, Lee JY, Wang KC. Secondary neurulation defects-1: Retained medullary cord. J Korean Neurosurg Soc. 2020. 63: 314-20
5. Kim KH, Lee JY, Yang J, Park SH, Kim SK, Wang KC. Cystic retained medullary cord in an intraspinal J-shaped cul-de-sac: A lesion in the spectrum of regression failure during secondary neurulation. Childs Nerv Syst. 2021. 37: 2051-6
6. Kurogi A, Murakami N, Morioka T, Mukae N, Shimogawa T, Kudo K. Two cases of retained medullary cord running parallel to a terminal lipoma. Surg Neurol Int. 2021. 12: 112
7. Morimoto K, Takemoto O, Nakamura H, Takeuchi M. Spinal dermal sinus associated with intramedullary abscess and dermoid. Pediatr Neurosurg. 2003. 39: 225-6
8. Morioka T, Murakami N, Shimogawa T, Mukae N, Hashiguchi K, Suzuki SO. Neurosurgical management and pathology of lumbosacral lipomas with tethered cord. Neuropathology. 2017. 37: 385-92
9. Morioka T, Murakami N, Kanata A, Tsukamoto E, Suzuki SO. Retained medullary cord with sacral subcutaneous meningocele and congenital dermal sinus. Childs Nerv Syst. 2020. 36: 423-7
10. Morioka T, Murakami N, Suzuki SO, Takada A, Tajiri S, Shimogawa T. Neurosurgical pathology and management of limited dorsal myeloschisis with congenital dermal sinus in infancy. Pediatr Neurosurg. 2020. 55: 113-25
11. Morioka T, Murakami N, Ichiyama M, Kusuda T, Suzuki SO. Congenital dermal sinus elements in each tethering stalk of coexisting thoracic limited dorsal myeloschisis and retained medullary cord. Pediatr Neurosurg. 2020. 55: 380-7
12. Morioka T, Murakami N, Suzuki SO, Mukae N, Shimogawa T, Kurogi A. Surgical histopathology of a filar anomaly as an additional tethering element associated with closed spinal dysraphism of primary neurulation failure. Surg Neurol Int. 2021. 12: 373
13. Morioka T, Murakami N, Suzuki SO, Nakamura R, Mizoguchi M. Subpial lumbar lipoma associated with retained medullary cord. NMC Case Rep J. 2021. 8: 51-5
14. Mukae N, Morioka T, Suzuki SO, Murakami N, Shimogawa T, Kanata A. Two cases of large filar cyst associated with terminal lipoma: Relationship with retained medullary cord. World Neurosurg. 2020. 142: 294-8
15. Murakami N, Morioka T, Shimogawa T, Hashiguchi K, Mukae N, Uchihashi K. Retained medullary cord extending to a sacral subcutaneous meningocele. Childs Nerv Syst. 2018. 34: 527-33
16. Murakami N, Morioka T, Shimogawa T, Mukae N, Inoha S, Sasaguri T. Ependyma-lined canal with surrounding neuroglial tissues in lumbosacral lipomatous malformations: Relationship with retained medullary cord. Pediatr Neurosurg. 2018. 53: 387-94
17. Pang D, Zovickian J, Moes GS. Retained medullary cord in humans: Late arrest of secondary neurulation. Neurosurgery. 2011. 68: 1500-19
18. Pang D, Chong S, Wang KC, di Rocco C, Pang D, Rutka JT.editors. Secondary neurulation defects-1: Thickened filum terminale, retained medullary cord. Textbook of Pediatric Neurosurgery. Switzerland: Springer; 2020. p.
19. Prasad GJ, Hegde A, Divya S. Spinal intramedullary abscess secondary to dermal sinus in children. Eur J Pediatr Surg. 2019. 29: 229-38
20. Sala F, Barone G, Tramontano V, Gallo P, Ghimenton C. Retained medullary cord confirmed by intraoperative neurophysiological mapping. Childs Nerv Syst. 2014. 30: 1287-91
21. Shirozu N, Morioka T, Inoha S, Imamoto N, Sasaguri T. Enlargement of sacral subcutaneous meningocele associated with retained medullary cord. Childs Nerv Syst. 2018. 34: 1785-90
22. Yang J, Lee JY, Kim KH, Wang KC. Disorders of secondary neurulation: Mainly focused on pathoembryogenesis. J Korean Neurosurg Soc. 2021. 64: 386-405