- Department of Neurosurgery, Hospital Angeles Culiacan, Sinaloa, Mexico.
- Department of Genomic Medicine Hospital General de Culiacan, Sinaloa, Mexico.
- Department of Pathology, Hospital General de Culiacan, Sinaloa, Mexico.
- Faculty of Medicine, Universidad Autonoma de Sinaloa, Culiacan, Sinaloa, Mexico.
Correspondence Address:
Jesus Rocha-Maguey
Faculty of Medicine, Universidad Autonoma de Sinaloa, Culiacan, Sinaloa, Mexico.
DOI:10.25259/SNI_215_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jesus Rocha-Maguey1, Jesus Salvador Velarde-Felix2, Myriam Cabrera-Lopez3, Jaime Moya-Nuñez4, Edgar Fragosa-Sanchez4. Intramedullary clear cell ependymoma of the lower thoracic spinal cord: report of a new case. 11-Dec-2020;11:423
How to cite this URL: Jesus Rocha-Maguey1, Jesus Salvador Velarde-Felix2, Myriam Cabrera-Lopez3, Jaime Moya-Nuñez4, Edgar Fragosa-Sanchez4. Intramedullary clear cell ependymoma of the lower thoracic spinal cord: report of a new case. 11-Dec-2020;11:423. Available from: https://surgicalneurologyint.com/surgicalint-articles/10453/
Abstract
Background: Clear cell ependymomas (CCEs) are a rare variant of tumors of the nervous system, the main location is the intracranial compartment. Special differential diagnosis should be done with oligodendrogliomas, neurocytoma, glioneurocytoma, astrocytoma, or metastatic renal cell carcinoma, lesions that somehow share cells with clear cytoplasm. Most of these lesions are benign but differential diagnosis is essential to decide further treatment. Few case reports of intramedullary CCEs have being published and there is no strict consensus on the diagnostic criteria.
Case Description: We hereby describe a new case of an intramedullary clear CCE with very few neurological symptoms, surgical treatment is satisfactory, histological and immunohistochemical analysis was confirmatory. After gross total resection and 3-year follow-up no recurrence of the lesion is evident.
Conclusion: After this case presentation and review of the limited literature, it is evident that methodical clinical suspicion, radiological imaging combined with histological, and modern immunohistochemical techniques are essential for the diagnosis. Surgical options with gross total resection remain the cornerstone of its treatment. Neurophysiological monitoring is extremely useful to avoid postoperative morbidity.
Keywords: Clear cell, Ependymoma, Intramedullary, Surgical treatment
INTRODUCTION
Clear cell ependymomas (CCEs) constitute a rare variant of tumors of the central nervous system. Most of them are located intracranially and specially in the supratentorial compartment. Spinal cord CCEs have being described previously and constitute a rare but very benign variant of intramedullary tumors.[
CASE REPORT
A 57-year-old Mexican woman was referred to neurosurgical consultation after being reviewed by the Clinical Neurology Department at our hospital. She assisted complaining with a 36-week history of moderate lower limbs pain, cramps, and gait disturbances, no sphincter problems were referred.
The initial neurologic evaluation demonstrated a dissociated sensory disturbance below Th11 level and decreased deep tendon reflex in both lower limbs. Strength was 4/5 on the left iliopsoas and quadriceps muscles associated with negative extensor plantar reflex. Gait disturbances were prominent preventing her to perform Tandem, Romberg sign and walking instability were evident as other proprioceptive alterations.
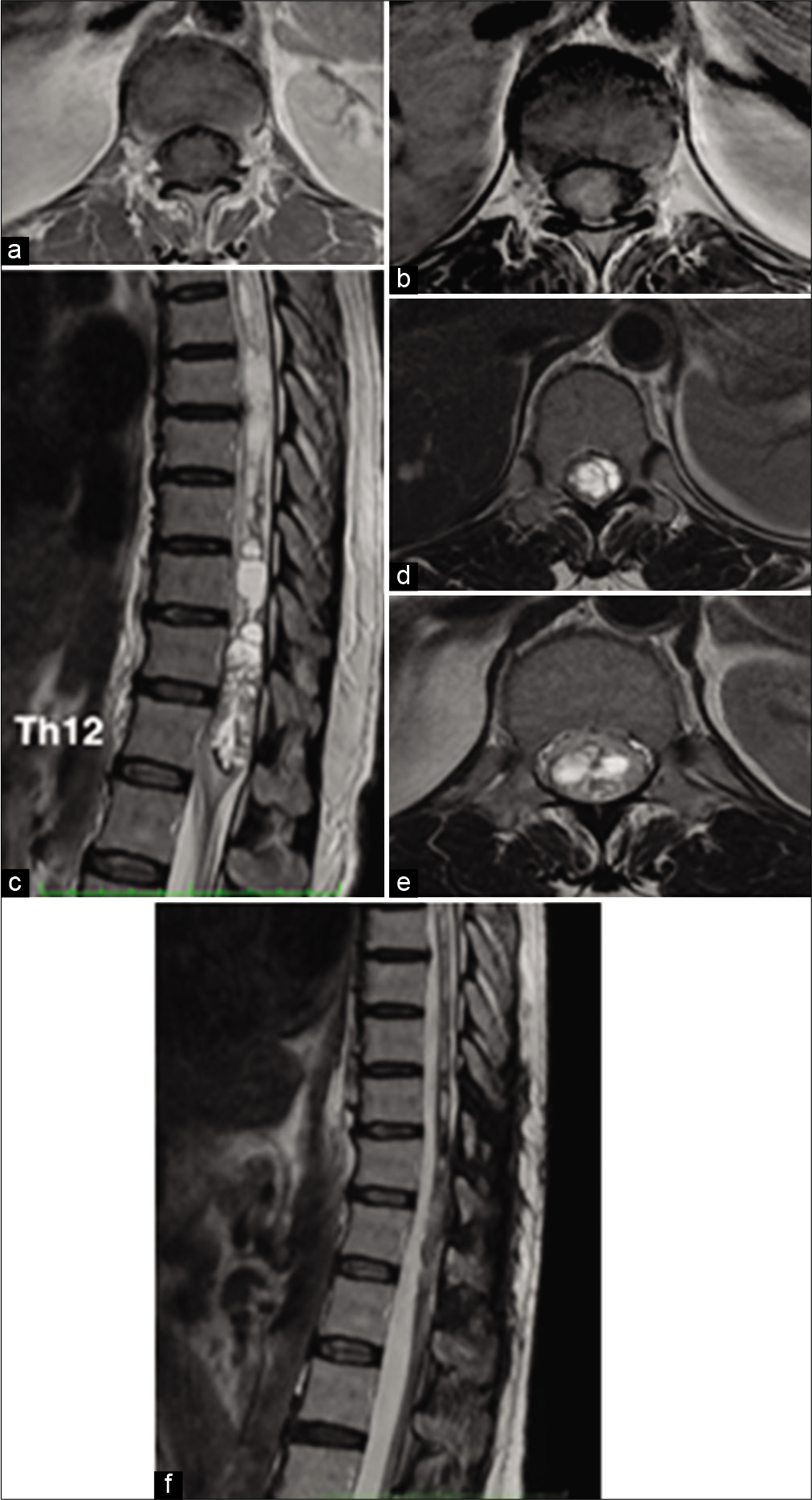
Magnetic resonance image (MRI) of the thoracolumbar spine [
Figure 1:
(a) Axial T1-W sequence demonstrate hypointense characteristics of the tumor expanding the thickness of the spinal cord. (b) Axial T1-W with Gd shows a moderate homogenous enhancement without a clear difference from the medullary tissue. (c) Sagittal view on T2-W sequence made clear that the tumor that extended from the superior border of Th11 to the inferior border of Th12. An adjacent cranial lobulated syrinx extending from Th11 to Th4 was observed. (d and e) Axial T2-W images of the spinal cord and filum terminals confirmed hyperintense characteristics of the tumor with a foamy-like characteristic and differentiated from spinal cord tissue. (f) Post-surgical sagittal T2-W sequence rules out tumor recurrence and decrease in the size of the syrinx (3 years later).
The patient was scheduled to a Th9-Th12 laminectomy, tumor resection, and laminoplasty, which was performed under neurophysiological monitoring with motor evoked potentials (MEP’s) and microsurgical techniques. After identifying the posterior medullary sulcus and performing a midline myelotomy, we could observe a highly vascularized grayish lesion that was firmly attached to the ependymal walls. Despite having a firm texture, no clear plane of cleavage was evident except in its upper pole where a cystic cavity containing xanthochromic fluid was emerging. An estimated 95% resection of the tumor could be achieved with ultrasonic aspiration assistance without eventualities. Medullary pia was slightly closed just to juxtapose the posterior columns using 7–0 non-absorbable stitches and allowing the syrinx to drain into the subarachnoid compartment. While the tumor was manipulated, a moderate and transient decrease in MEP’s amplitude was detected, however progressive recovery of the registry allowed us to continue with the procedure. Postsurgical evolution was satisfactory and the patient did not show any increase of her previous neurological manifestations. After the 5th postoperative day, she was discharged from the hospital to her home under a physical therapy program for her legs. Tumor recurrence is discarded after 24 months of follow-up MRI [
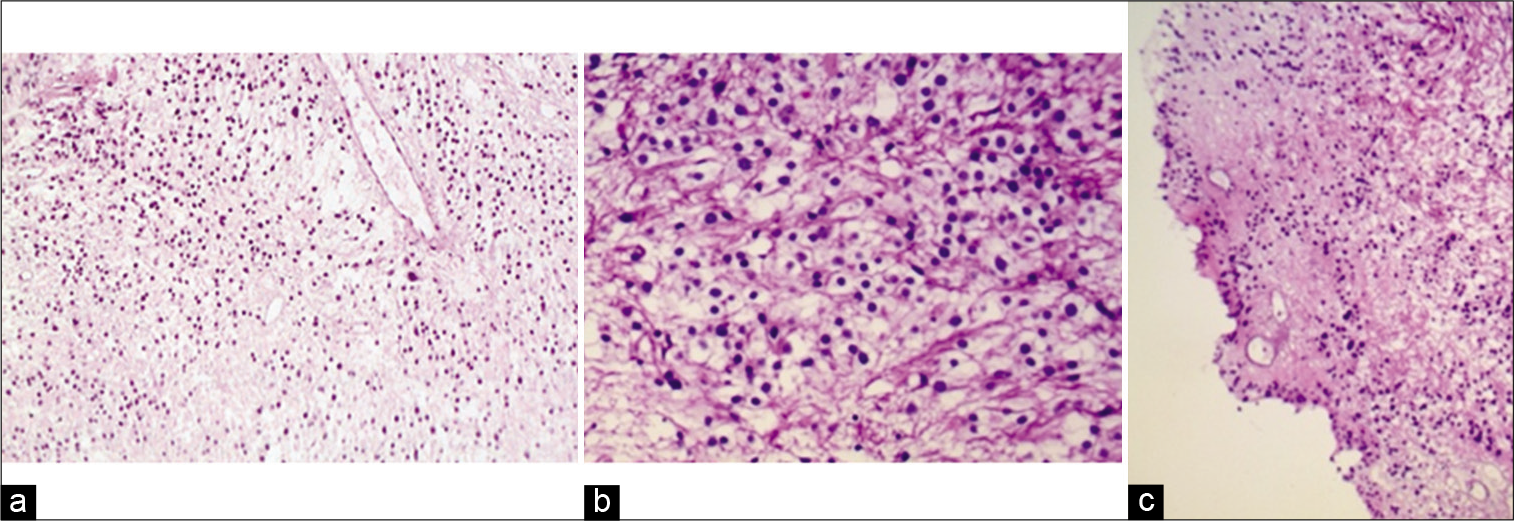
For histological purposes, 3–4 μm-thick sections were stained with hematoxylin and eosin, finding an extensive proliferation of monotonic round to oval cells with clear fibrillary cytoplasm and large central round nucleus with indistinct nucleoli disposed in a diffuse pattern and scarce mitotic activity than normal tissues. Entrapped blood vessels showed moderate perivascular hyalinization and the presence of pseudorosettes suggesting; thus, the diagnosis of CCE without anaplasia [
Figure 2:
(a) Panoramic photomicrograph (×10) demonstrating proliferation of monotone cellularity with clear cytoplasm and round nuclei. (b) (×40) Most of the tumoral cells show a high nuclear-to cytoplasm ratio with round or slightly oval nuclei and a clear perinuclear halo. (c) Pseudorosettes, as perivascular cuffs of tumoral cells with processes oriented towards the central vessel are visualized in a non-organized mode (H and E Stain).
DISCUSSION
Ependymoma is a group of tumors that have their origin from the ependymal cells of the ventricular system of the brain and in the central canal of the spinal cord. Most of intracranial cases are predominant in childhood, whereas the intramedullary cases usually occur in adults with a characteristic preference for the cervical and thoracic regions in the spinal cord.[
It is well recognized that most ependymomas are usually of benign etiology and their symptoms derive from the neurological deficit caused by the underlying affected structures without presenting infiltrative characteristics. Cysts associated with intramedullary ependymomas are frequent, their presence is near to 80% of cases and it is considered that the volume of the cysts is proportional to the severity of symptoms presented by patients.[
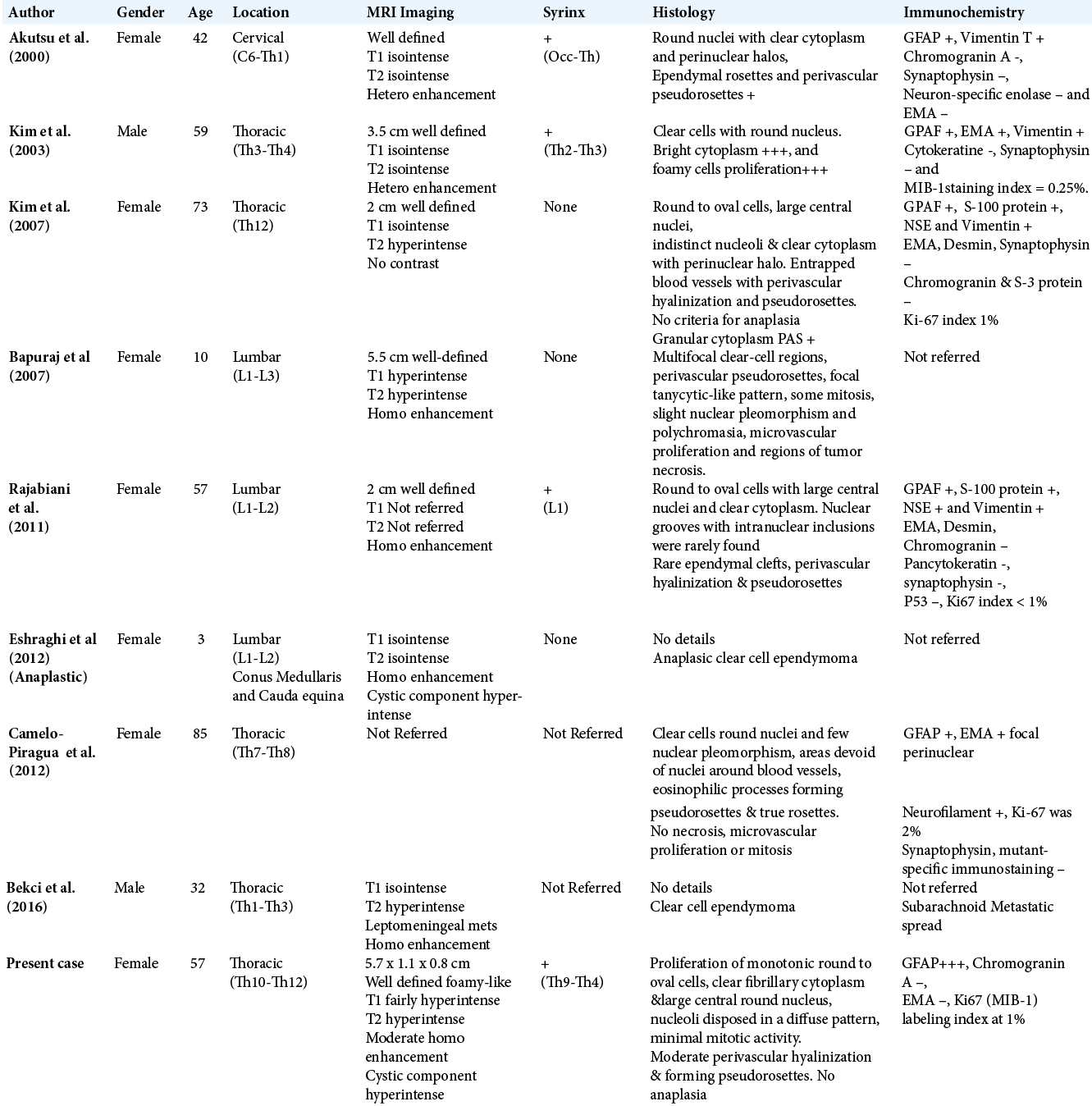
The predominant location is the thoracic region, followed by high lumbar and the cervical segments (5, 3, and 1 cases, respectively). Akutsu et al. in 2000, reported the only patient with cervical location to date, it is about a female that experienced ulnar numbness and moderate spastic weakness of the lower limbs.[
Concerning their morphology, normal ependymal cells can vary considerably ranging from ciliated or cuboidal epithelial cells to elongate and fibrillated glial cells also known as tanycytes. The same is true for ependymomas, resulting in three histopathological variants: papillary ependymoma (PE), CCE, and tanycytic ependymoma being this last the most recently recognized ependymoma variant.[
Histologically, ependymoma shows characteristic diagnostic perivascular pseudorosettes and/or true rosettes called ependymal rosettes and ependymal tubes or canals.[
Clear cells or oligodendroglia-like cells have been noticed in ependymomas for many years. The CCE was first described by Kawano et al. and has been practically restricted to the supratentorial compartment with clinical and biological behavior that may not differ from other ependymal lesions.[
Microscopically, these tumors are composed by a moderately round to oval cell population with large central round nuclei and clear cytoplasm, but without atypical features. Nuclear grooves and intranuclear inclusions are rarely found in the specimens but a very well-defined fibrillary background can be easily noticed in certain portions of tumor. Rare ependymal clefts, perivascular hyalinization and pseudorosettes may also be identified.[
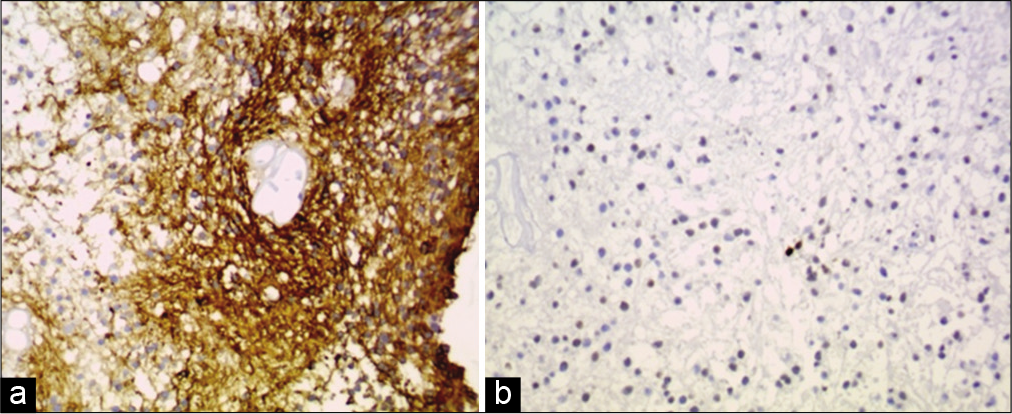
In a review and analysis of different clear-cell tumors of the central nervous system, an adequate identification of the origin of these neoplasms is possible by combining GFAP, EMA, and synaptophysin.[
AE1/AE3. Neuronal markers including synaptophysin, neurofilaments, neuron-specific enolase, and chromogranin A are usually negative, and as very distinctive characteristic is that most of them show a low Ki-67 (MIB-1) labeling index even at the very positive immunoreactive protein areas and very few p53-positive cells.[
Although very strict immune reactive characteristics have been well defined for ependymomas, electron microscopy (EM) has showed some specific diagnostic peculiarities, highlighting complex intercellular junctions, surface microvilli, and cilia as well as micro rosettes formation. However, lack of secretory granules, vesicles, absence of peripheral parts of the cytoplasm including organelles, and synapses may appeared to be electron lucent and suggestive of cellular edema, which might explain the clear cell features, so the use of EM should be considered in questionable situations.[
Radiologically, unlike the cellular or myxopapillary spinal cord ependymomas, CCEs are homogeneously isointense to hyperintense on T1-W images and hyperintense on T2-W images, sometimes showing homogeneous enhancement after gadolinium administration and their margins are well defined and they appear nonaggressive.[
Amatya et al. described the lesion as “cystic,” because the tumor appeared to have solid as well as cystic components on MR images. The isodense solid component enhanced uniformly while the cystic component did not. T2-W sequences were clearly hyperintense with irregular borders but well defined from the spinal cord tissue.[
Surgical management is by far the best option of treatment for these neoplasms. The goal of the treatment is focused on gross total excision preserving surrounding tissues; good surgical results are achievable through radical resection with minimal resultant morbidity.[
Maintenance of the capsule is an important component of tumor excision to obtain an in-bloc resection, a careful circumferential dissection of the spinal cord interphase is performed, this may decrease the chances for a tumor dissemination simultaneously.[
Routinely, we attempt to decrease in postoperative morbidity using neurophysiologic monitoring during surgery. Prevention of injury to motor pathways and further neurological deficit can be achieved with continue MEP monitoring, while the spinal cord is operated. Although somatosensory evoked potential monitoring has not predicted functional outcomes, we usually monitor them while the dorsal midline myelotomy is performed. [
If a total resection is achieved, no further therapy with radiation or chemotherapy is necessary. Surgical options are not different from other intramedullary tumors, but a great effort is sometimes required to obtain the most extensive resection to avoid a possible recurrence.[
CONCLUSION
With few reported cases, the diagnosis of CCEs requires very straight neuroimaging, histologic and ultrastructural correlation, especially when limited biopsy fragments are available. Current immunohistochemical techniques demonstrate very specific parameters for the differential diagnosis of clear-cell tumors. Radical surgical resection is considered the optimal treatment option. Satisfactory surgical outcome depends on adequate pre-operative planning, fine operative manipulation, and continuous intra-operative monitoring. Determining an adequate plane of cleavage allows preservation of functional spinal cord tissue. Despite being considered as benign lesions, radiotherapy should be considered in those cases with malignant characteristics or with evidence of meningeal dissemination. The importance of an adequate recognition is the avoidance of alternative misdiagnosis and inappropriate therapies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Akutsu H, Shibata Y, Okazaki M, Hyodo A, Matsumura A. Intramedullary clear cell ependymoma in the cervical spinal cord: Case report. Neurosurgery. 2000. 47: 1434-8
2. Amatya VJ, Takeshima Y, Kaneko M, Nakano T, Yamaguchi S, Sugiyama K. Case of clear cell ependymoma of medulla oblongata: Clinicopathological and immunohistochemical study with literature review. Pathol Int. 2003. 53: 297-302
3. Bapuraj JR, Parmar HA, Blaivas M, Muraszko KM. Imaging features of clear-cell ependymoma of the spinal cord. Pediatr Radiol. 2007. 37: 384-7
4. Bekci T, Aslan K, Gunbey HP, Sayit AT, Incesu L. Clear cell ependymoma with late leptomeningeal and supratentorial metastases. J Spine. 2016. 16: e47
5. Camelo-Piragua S. Clear cell tumors of the central nervous system. A case-based review. Arch Pathol Lab Med. 2012. 136: 915-26
6. Celano E, Salehani A, Malcolm JA, Reinertsen E, Hadjipanayis CG. Spinal cord ependymoma: A review of the literature and case series of ten patients. J Neurooncol. 2016. 128: 377-86
7. Costa P, Peretta P, Faccani G. Relevance of intraoperative D wave in spine and spinal cord surgeries. Eur Spine J. 2013. 22: 840-8
8. Daulac C, Messerer R, Obadia-Andre N, Afathi M, Barrey CY. Cysts associated with intramedullary ependymomas of the spinal cord: Clinical, MRI and oncological features. J Neurooncol. 2019. 144: 385-91
9. Eshraghi H, Andronikou S, Fisher-Jeffes N, Truter R, Walt AV. Anaplastic clear cell ependymoma of the conus medullaris and cauda equina in a 3-year-old child. J Pediatr Hematol Oncol. 2012. 34: 316-7
10. Giammattei L, Penet N, Parker F, Messerer M. Intramedullary ependymoma: Microsurgical resection technique. Neurochirurgie. 2017. 63: 398-401
11. Ho D, Hsu C, Wong T, Chiang H. A clinicopathologic study of 81 patients with ependymomas and proposal of diagnostic criteria for anaplastic ependymoma. J Neurooncol. 2001. 54: 77-85
12. Karikari IO, Nimjee SM, Hodges TR, Cutrell E, Hughes BD, Powers CJ. Impact of tumor histology on resectability and neurological outcome in primary intramedullary spinal cord tumors: A single-center experience with 102 patients. Neurosurgery. 2011. 68: 188-97
13. Kawano N, Yada K, Yagishita S. Clear cell ependymoma. A histological variant with diagnostic implications. Virchows Arch A Pathol Anat Histopathol. 1989. 415: 467-72
14. Kim YJ, Tsunoda SH, Yokoyama K, Miyamoto K, Tamai M, Yamauchi M. Clear cell ependymoma with a lipidized component that developed in the thoracic spinal cord. Neurol Res. 2003. 25: 324-8
15. Kim NR, Chung DH, Lee SK, Ha SY. Intramedullary clear cell ependymoma in the thoracic spinal cord: A case with its crush smear and ultrastructural findings. J Korean Med Sci. 2007. 22: S149-53
16. Klekamp J. Spinal ependymomas. Part 1: Intramedullary ependymomas. Neurosurg Focus. 2015. 39: E6
17. Koperek O, Gelpi E, Birner P, Haberler C, Budka H, Hainfellner JA. Value and limits of immunohistochemistry in differential diagnosis of clear cell primary brain tumors. Acta Neuropathol. 2004. 108: 24-30
18. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803
19. Min KW, Scheithauer BW. Clear cell ependymoma: A mimic of oligodendroglioma: Clinicopathologic and ultrastructural considerations. Am J Surg Pathol. 1997. 21: 820-6
20. Mohammed W, Farrell M, Bolger C. Spinal cord ependymoma-surgical management and outcome. J Neurosci Rural Pract. 2019. 10: 316-20
21. Nakamura M, Ishii K, Watanabe K, Tsuji T, Takaishi H, Matsumoto M. Surgical treatment of intramedullary spinal cord tumors: Prognosis and complications. Spinal Cord. 2008. 46: 282-6
22. Nagasawa DT, Smith ZA, Cremer N, Fong C, Lu DC, Yang I. Complications associated with the treatment for spinal ependymomas. Neurosurg Focus. 2011. 31: E13
23. Ottenhausen M, Ntoulias G, Bodhinayake I. Intradural spinal tumors in adults-update on management and outcome. Neurosurg Rev. 2019. 42: 371
24. Rajabiani A, Saffar H, Kamalian N, Rajabiani A, Saffar H, Kamalian N. Clear cell ependymoma of spinal cord: A case report. Iran J Pathol. 2011. 6: 36-40
25. Rosenblum MK. Ependymal tumors: A review of their diagnostic surgical pathology. Pediatr Neurosurg. 1998. 28: 160-5
26. Sala F, Palandri G, Basso E, Lanteri P, Deletis V, Faccioli F. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: A historical control study. Neurosurgery. 2006. 58: 1129-43
27. Seyithanoglu H, Guzey FK, Emel E, Alatas I, Acarbas A, Ozkan N. Clear cell ependymoma of the temporal lobe in a child: A case report. Pediatr Neurosurg. 2008. 44: 79-84
28. Traul DE, Shaffrey ME, Schiff D. Part I: Spinal-cord neoplasmsintradural neoplasms. Lancet Oncol. 2007. 8: 35-45
29. Tredway TL. Minimally invasive approaches for the treatment of intramedullary spinal tumors. Neurosurg Clin N Am. 2014. 25: 327-36
30. Vandertop WP. Spinal cord ependymoma: Radical surgical resection and outcome. Neurosurgery. 2003. 53: 246
31. Wiestler OD, Schiffer D, Coons SW, Prayson RA, Rosenblum MK, Kleihaues P, Cavenee WK.editors. Ependymoma. World Health Organization Classification of Tumours, Pathology and Genetics, Tumours of the Nervous System. Lyon, France: IARC Press; 2000. p. 72-6