- Department of Neurosurgery, University of Passo Fundo, Passo Fundo, Rio Grande do Sul, Brazil,
- Department of Neurosurgery, São Vicente de Paulo Hospital, Passo Fundo, Rio Grande do Sul, Brazil,
- Department of Spine Surgery, Clínica de Cuyo, Mendoza, Argentina.
Correspondence Address:
Eduardo Cattapan Piovesan, Department of Neurosurgery, University of Passo Fundo, Passo Fundo, Rio Grande do Sul, Brazil.
DOI:10.25259/SNI_399_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Eduardo Cattapan Piovesan1, Werner Petry Silva2, Adroaldo Baseggio Mallmann2, Alfredo José Guiroy3, Charles André Carazzo1,2. Intramedullary histoplasmosis of the thoracic cord as an isolated lesion: A rare case report and literature review. 08-Jun-2023;14:197
How to cite this URL: Eduardo Cattapan Piovesan1, Werner Petry Silva2, Adroaldo Baseggio Mallmann2, Alfredo José Guiroy3, Charles André Carazzo1,2. Intramedullary histoplasmosis of the thoracic cord as an isolated lesion: A rare case report and literature review. 08-Jun-2023;14:197. Available from: https://surgicalneurologyint.com/surgicalint-articles/12358/
Abstract
Background: Disseminated histoplasmosis involving the central nervous system occurs in 5–10% of cases. However, intramedullary spinal cord lesions are extremely rare. Here, 45-year-old female with a T8–9 intramedullary lesion did well following surgical extirpation.
Case Description: For 2 weeks, a 45-year-old female experienced progressive lower back pain, paresthesias, and paraparesis. The magnetic resonance imaging showed an intramedullary expansive lesion at the T8–T9 level that markedly enhanced with contrast. Surgery, consisting of T8–T10 laminectomies performed using neuronavigation, an operating microscope, and intraoperative monitoring, revealed a well-demarcated lesion that proved to be a focus of histoplasmosis; it was readily completely excised.
Conclusion: Surgery is the gold standard for treating spinal cord compression attributed to intramedullary histoplasmosis unresponsive to medical management.
Keywords: Histoplasmosis, Neurosurgery, Spine infection, Spine surgery, Spine
INTRODUCTION
Disseminated histoplasmosis involves the central nervous system (CNS) in 5–10% of cases, but only rarely is found within the spinal cord itself (i.e., intramedullary/intradural). The most common neurological symptoms/signs correlate with the various brain and spinal lesion locations, while other findings may include chronic meningitis, hydrocephalus, and/or encephalitis. Approximately 1/3 of cases occur in immunocompetent versus 2/3 in immunosuppressed individuals.[
CASE REPORT
Clinical presentation
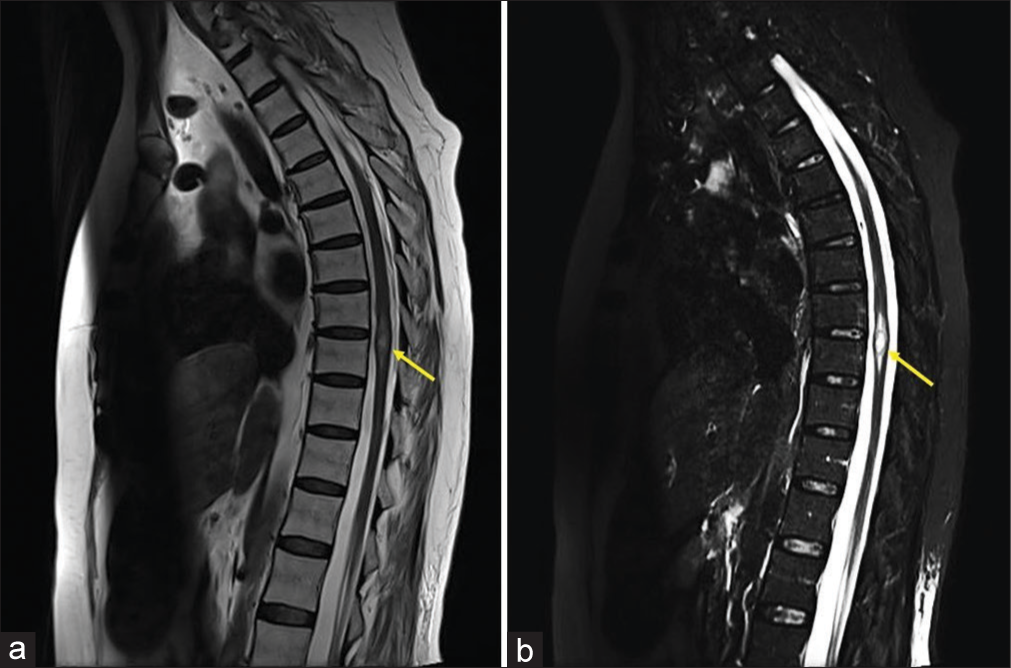
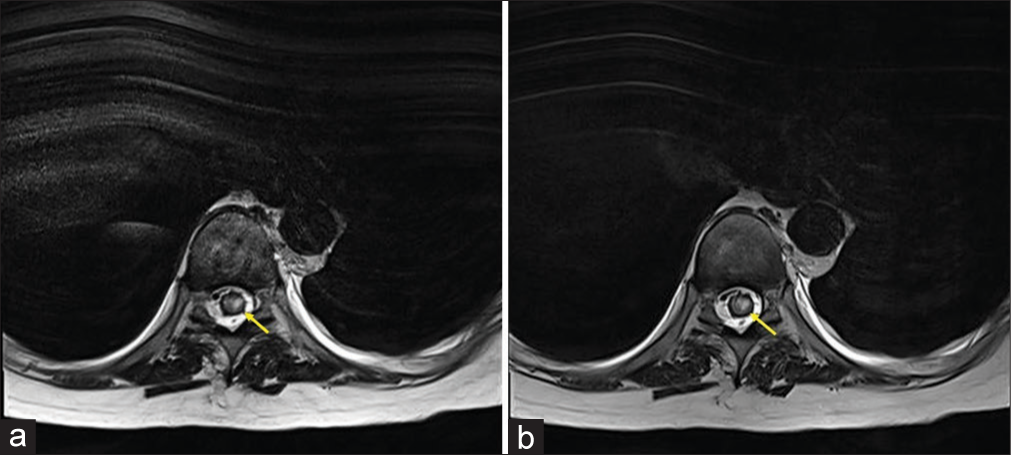
An immunocompetent 45-year-old female presented with a 2-week history of progressive lower back pain, paresthesias, and paraparesis. Within a few days, the patient progressed from 3/5 to 1/5 lower extremity weakness; her neurological findings also included diffuse hyperreflexia, bilateral Babinski signs, and urinary retention. The magnetic ressonance imaging (MRI) of the spine demonstrated an intramedullary expansive lesion at the T8–T9 level that markedly enhanced with contrast [
Figure 1:
Magnetic resonance imaging (MRI) of the thoracic spine. (a) Sagittal T2-weighted revealed an intramedullary expansive lesion (yellow arrow). (b) Sagittal T2-weighted short-tau inversion recovery (STIR) image revealing an intramedullary intradural hyperintense mass (yellow arrow) at the T8–T9 level.
Surgery
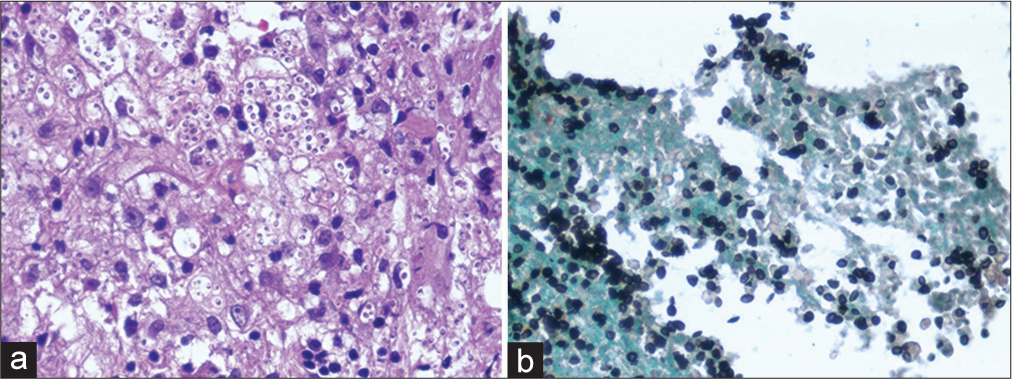
Utilizing neuronavigation, an operative microscope, and intraoperative monitoring, the patient underwent a T8–10 laminectomy. As soon as the dura and arachnoid were opened, an expansile intramedullary cord lesion was visualized, and xanthochromic/purulent fluid immediately drained under increased pressure. A well-demarcated cleavage plane between the lesion and cord facilitated gross-total lesion resection. The pathological analysis was diagnostic for histoplasmosis [
Figure 3:
Histology of intramedullary histoplasmosis lesion. (a) Hematoxylin and eosin (H&E) staining revealed inflammatory process, where epithelioid macrophages, lymphocytes, and multinucleated giant cell are observed. (b) Grocott methenamine silves (GMS) staining identified fungal microorganisms that are morphologically consistent with histoplasma.
DISCUSSION
Definition and frequency of isolated versus disseminated histoplasmosis
Histoplasmosis is defined as an intracellular infection caused by the fungus histoplasma capsulatum. The first case involving the CNS was described in an infant in 1934.[
Pathogenesis of spinal intramedullary histoplasmosis
Intramedullary spinal histoplasmosis is extremely rare, and the literature suggests that 50–90% of immunocompetent individuals with histoplasmosis remain asymptomatic. Symptomatic cases are often observed in immunocompromised patients, and the signs/symptoms correlate with the location of brain or spine lesions (i.e., myelopathy). Other findings include; chronic meningitis, cerebral vasculitis/stroke syndromes, hydrocephalus (chronic recurrent), and encephalitis [
MRI of intramedullary histoplasmosis
MRI findings for intramedullary histoplasmosis include focal nodular low-intensity T1 lesions, and high-intensity T2 lesions that heterogeneously enhance. The lesion is nodular or like a ring one and, if a lesion is found, the entire neuraxis should be assessed.[
Delayed diagnosis of CNS histoplasmosis utilizing antibody/antigen testing, cultures, and biopsy
The diagnosis of CNS histoplasmosis is challenging and typically delayed.[
Morbidity and mortality for CNS histoplasmosis
Patients with CNS histoplasmosis exhibit high morbidity, mortality, and relapse rates.[
Medical management of CNS histoplasmosis
For those without CNS involvement not requiring hospitalization, itraconazole is the treatment of choice. For those hospitalized or with CNS involvement, initial therapy should include amphotericin B with transitioning to itraconazole.[
Medical and/or surgical management of CNS histoplasmosis
Fewer than 50% of cases of CNS histoplasmosis are cured by antifungal treatment alone. Therefore, the treatment of choice for those patients, particularly with progressive neurologic deficit/deterioration despite medical management, includes amphotericin B and surgical lesion resection.[
CONCLUSION
CNS histoplasmosis can be difficult to diagnose due to its atypical features and because it can mimic other systemic diseases. CNS histoplasmosis should be considered even in non-endemic areas, with or without a history of immunosuppression, and even with isolated spinal cord injury. In the present study, a 45-year-old female presented with myelopathy/paraparesis attributed to a T8–T9 intramedullary histoplasmosis that presented as an isolated lesion. Following complete lesion resection, the patient recovered the neurological function.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Gonzalez HH, Rane M, Cioci A, Goodman S, Espinosa PS. Disseminated central nervous system histoplasmosis: A case report. Cureus. 2019. 11: e4238
2. Hariri OR, Minasian T, Quadri SA, Dyurgerova A, Farr S, Miulli DE. Histoplasmosis with deep CNS involvement: Case presentation with discussion and literature review. J Neurol Surg Rep. 2015. 76: 167-72
3. Hott JS, Horn E, Sonntag VK, Coons SW, Shetter A. Intramedullary histoplasmosis spinal cord abscess in a nonendemic region: Case report and review of the literature. J Spinal Disord Tech. 2003. 16: 212-5
4. Manning TC, Born D, Tredway TL. Spinal intramedullary histoplasmosis as the initial presentation of human immunodeficiency virus infection: case report. Neurosurgery. 2006. 59: 1160-3
5. N’dri-Oka D, Adou N, Tokpa A, Derou L. Intramedullary spinal cord compression caused by Histoplasma capsulatum A case report and meta-analysis. J Neurosurg. 2015. 4: 109-13
6. Recker MJ, Housley SB, Lipinski LJ. Indolent nonendemic central nervous system histoplasmosis presenting as an isolated intramedullary enhancing spinal cord lesion. Surg Neurol Int. 2021. 12: 392
7. Renan J, Filho MC, Rodrigues P, Spir N, Cortez GM, Malveira AS. Intramedullary histoplasmosis lesion in children: A case report. Surg Neurol Int. 2022. 13: 83
8. Riddell J, Wheat LJ. Central nervous system infection with Histoplasma capsulatum. J Fungi (Basel). 2019. 5: 70
9. Simms A, Kobayashi T, Endelman L, Sekar P. Disseminated histoplasmosis presenting as bilateral lower extremity paresis. Int J Infect Dis. 2020. 95: 265-7
10. Voelker JL, Muller J, Worth RM. Intramedullary spinal Histoplasma granuloma Case report. J Neurosurg. 1989. 70: 959-61
11. Wheat J, Myint T, Guo Y, Kemmer P, Hage C, Terry C. Central nervous system histoplasmosis: Multicenter retrospective study on clinical features, diagnostic approach and outcome of treatment. Medicine (Baltimore). 2018. 97: e0245
12. Wheat LJ, Musial CE, Jenny-Avital E. Diagnosis and management of central nervous system histoplasmosis. Clin Infect Dis. 2005. 40: 844-52