- Department of Neurosurgery, Shin-Kuki General Hospital, Kuki, Saitama, Japan,
- Department of Neurosurgery, Juntendo Urayasu Hospital, Urayasu, Chiba, Japan,
- Department of Neurosurgery, Juntendo University, Hongo, Tokyo, Japan.
Correspondence Address:
Hiroshi Kageyama
Department of Neurosurgery, Juntendo University, Hongo, Tokyo, Japan.
DOI:10.25259/SNI_466_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hiroshi Kageyama, Takamoto Suzuki, Yukou Ohara. Intramedullary spinal cord germinoma clinically mimicking multiple sclerosis: A case report. 11-Oct-2019;10:201
How to cite this URL: Hiroshi Kageyama, Takamoto Suzuki, Yukou Ohara. Intramedullary spinal cord germinoma clinically mimicking multiple sclerosis: A case report. 11-Oct-2019;10:201. Available from: http://surgicalneurologyint.com/surgicalint-articles/9695/
Abstract
Background: It is important to differentiate intramedullary neoplastic lesions from nonneoplastic diseases such as multiple sclerosis (MS) and other demyelinating or inflammatory diseases.
Case Description: A 26-year-old Japanese male presented with a history of intracranial germinomas and obstructive hydrocephalus, treated with endoscopic surgery, and adjuvant chemotherapy and radiation therapy. Three years later, he developed paresthesias involving the right hand and both lower extremities. The cervical MR scan demonstrated a heterogeneously enhancing intramedullary C1-C2 lesion with surrounding edema. On cytological examination of the cerebrospinal fluid (CSF), there were no neoplastic cells. However, the fluid was positive for oligoclonal immunoglobulin G (IgG) bands. The patient received steroid pulse therapy to address the potential MS diagnosis. The follow-up MR showed reduced edema, but no change in the size of the intramedullary lesion. Therefore, the patient underwent a cervical laminectomy for tumor resection. The pathology was consistent with the same cranial germinoma treated 3 years previously. He subsequently received whole spinal radiation and three courses of chemotherapy.
Conclusion: Some spinal cord tumors may produce oligoclonal IgG bands in CSF. In this case, an intramedullary C1-C2 spinal cord germinoma was originally misdiagnosed as MS due to the presence of oligoclonal IgG bands in CSF. Differentiating this tumor from MS and initiating appropriate treatment were critical into the care of this patient.
Keywords: Germinoma, Multiple sclerosis, Oligoclonal band immunoglobulin G, Spinal cord tumor
INTRODUCTION
It is important to differentiate intramedullary neoplastic lesions from nonneoplastic diseases such as multiple sclerosis (MS) and other demyelinating or inflammatory diseases. Here, we report a drop metastasis from a cranial germinoma, resulting in an intramedullary C1-C2 cervical tumor documented on an enhanced MR. It was notably difficult in distinguishing this intramedullary metastatic germinoma from a potential MS lesion as the cerebrospinal fluid (CSF) was positive for oligoclonal immunoglobulin G (IgG) bands.
CASE DESCRIPTION
Original presentation
A 26-year-old Japanese male presented with headaches, anorexia, and diplopia. The enhanced computed tomography scan showed two small intracranial masses; one was a suprasellar lesion and the other appeared at the aperture of the aqueduct, resulting in obstructive hydrocephalus. No lesions were found in the spinal cord. An endoscopic biopsy was performed of the suprasellar mass, and the accompanying third ventriculostomy resolved the hydrocephalus. The pathology revealed a germinoma and he received three courses of chemotherapy (carboplatin, 450 mg for 1 day; etoposide, 1100 mg for 5 days). This was followed by whole-brain radiation (24 Gy). Ultimately, the intracranial lesions disappeared.
New intramedullary lesion 3 years later
Three years later, however, the patient experienced vacillating paresthesia in his right hand and both legs, but without a focal neurological deficit. Human chorionic gonadotropin β-subunit (βhCG) and α-fetoprotein (AFP) were within normal limits in the serum (βhCG <0.1 ng/ml and AFP 2.2 ng/ml), CSF βhCG was 0.4 ng/ml, and AFP was 0.2 ng/ml. The cytological examination of CSF was negative. However, oligoclonal IgG bands were positive in CSF (IgG index, 0.66; myelin basic protein, 45.8 pg/ml).
Radiological diagnostic evaluation
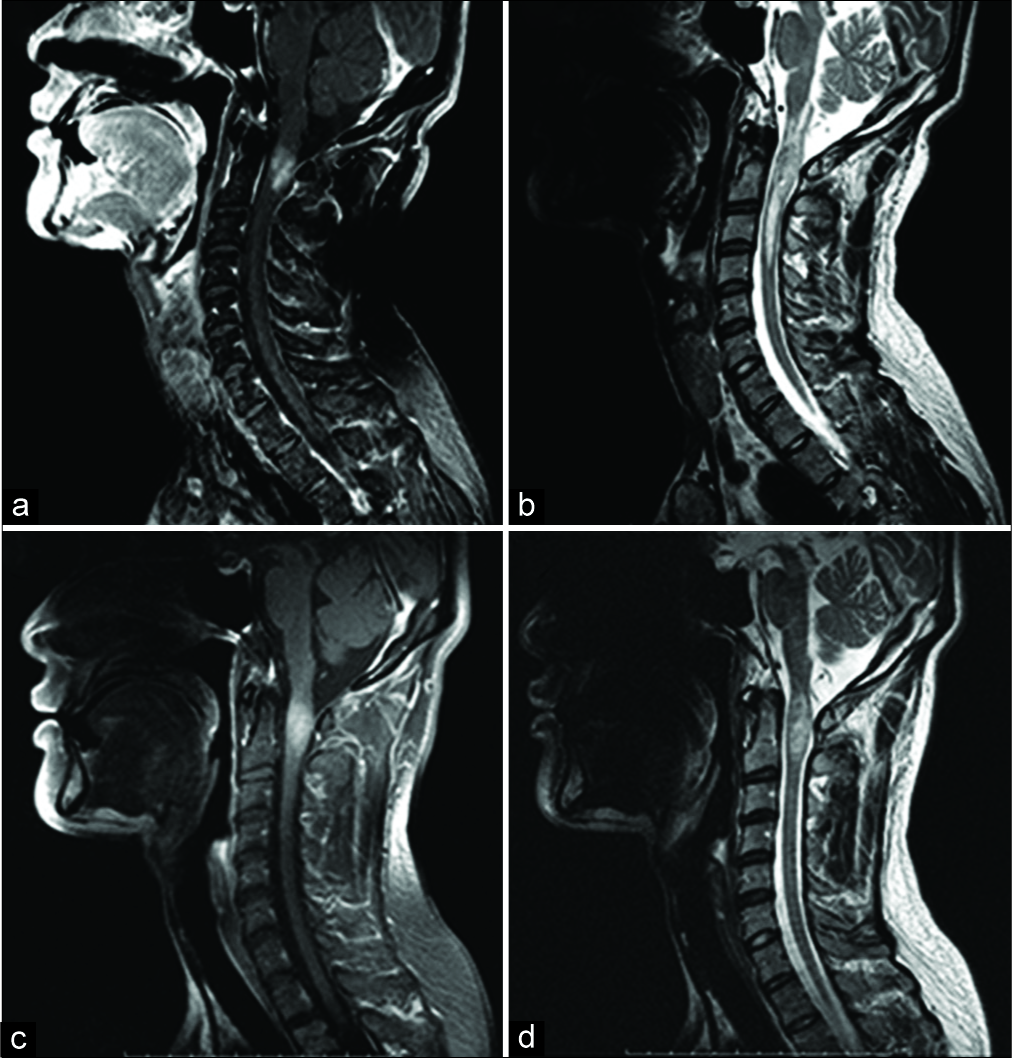
The cervical MR revealed a heterogeneously enhancing, expansile intramedullary cord lesion at the C1-C2 level, accompanied by marked edema extending from the medulla oblongata to the C4 level [
Figure 1:
(a) Sagittal T1-weighted postgadolinium magnetic resonance (MR) images through the cervical spine showing intense contrast enhancement of an intramedullary lesion from the C1 to C2 level. (b) Sagittal T2-weighted MR images demonstrating the heterogeneous intramedullary lesion extending from the medulla oblongata to the C4 level, which was thought to represent spinal cord edema surrounding the enhanced mass. (c and d) Scans after steroid pulse therapy. (c) Sagittal T1-weighted postgadolinium MR images showing no change in the enhanced lesion. (d) Sagittal T2- weighted MR images showing a decrease in cord edema.
Differential diagnosis and treatment
The main differential diagnoses included; astrocytoma, ependymoma, or germinoma along with other nonneoplastic diseases (e.g., MS, other demyelinating diseases, or inflammatory myelitis). Due to the potential diagnosis of MS, the patient received steroid pulse therapy with methylprednisolone (1 g/day) for 3 days. The more likely diagnosis of a tumor was later confirmed when the follow-up magnetic resonance imaging (MRI) showed reduced edema around the unchanged contrast-enhancing C1-C2 intramedullary mass [
Surgery
One month later, the patient underwent a C1 laminectomy/ C2 partial laminectomy with revised laminoplasty of the C2 spinous process for resection of the intramedullary cervical lesion. A myelotomy was performed along the posterior median sulcus; just under the cord surface, the tumor was grayish, soft, and nonhemorrhagic and appeared to grow into the central canal. As the intraoperative frozen section diagnosis was consistent with germinoma, a sufficient biopsy/decompression was performed without the need for total resection.
Pathology
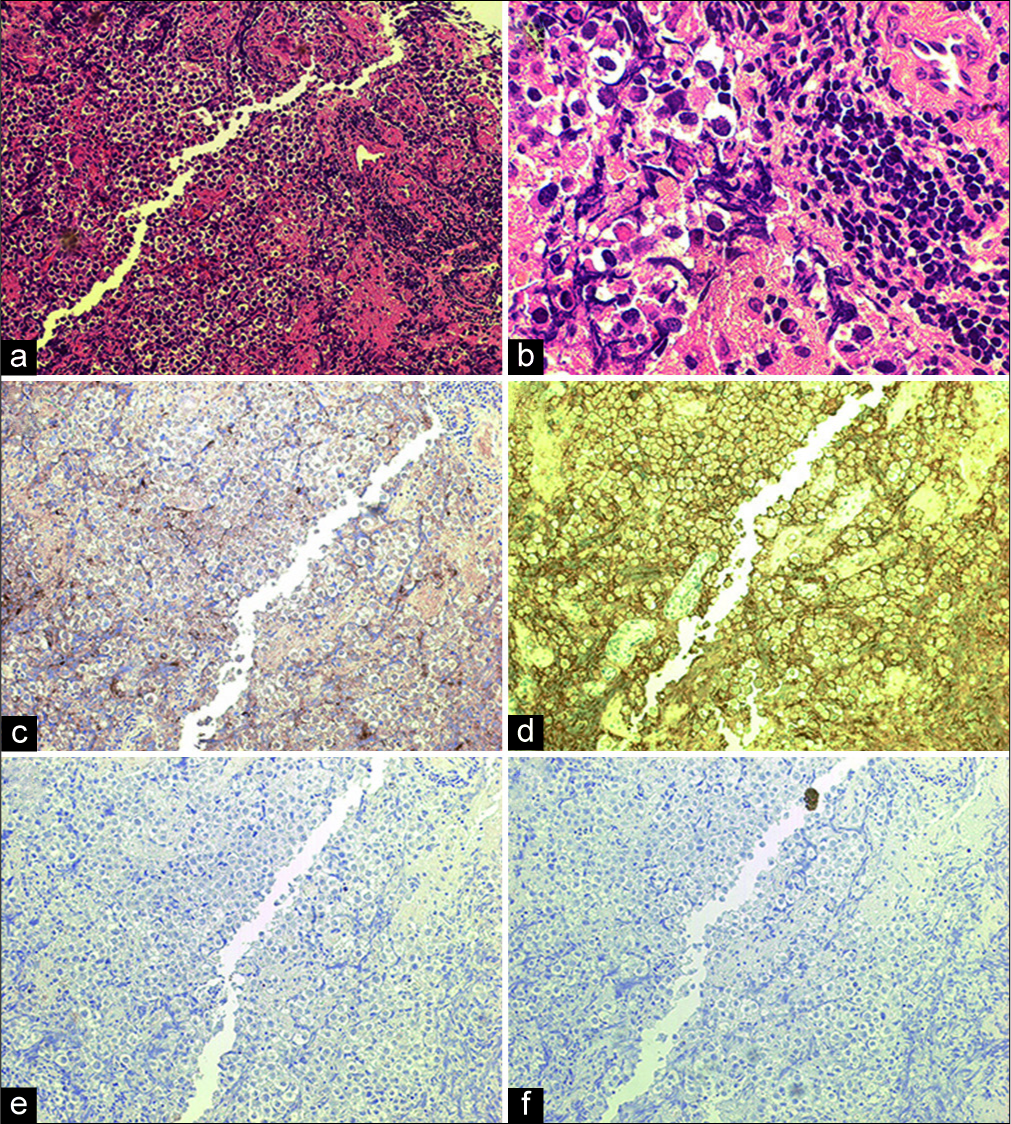
The tumor consisted of a mixed population of large cells (e.g., large round nuclei, hyperchromasias, with clear cytoplasm) and mature lymphocytes [two-cell pattern,
Figure 2:
(a) Photomicrographs of the operative specimen at low power (original, ×100) displaying a mixed population of large atypical cells and small lymphocytes (two-cell pattern). Large atypical cells had proliferated with a solid to sheet-like pattern. The small lymphocytes had invaded the stroma. There were no syncytiotrophoblastic giant cells. (b) High-power micrograph of the tumor (original, ×400) displaying large round cells characterized by nuclear atypia with hyperchromatic large ovale and a clear cytoplasm. Invading lymphocytes were matured. (c-f) Immunohistochemical stains of the tumor. The large atypical cells were diffusely positive for placental alkaline phosphatase and C-kit (original, ×100). (c) Placental alkaline phosphatase. (d) C-kit. (e) α-fetoprotein. (f) Human chorionic gonadotropin.
DISCUSSION
Differential diagnosis tumor versus MS
For intramedullary spinal cord lesions, it is important to differentiate tumors from nonneoplastic diseases (e.g., MS, demyelinating disease, acute and subacute myelitis [viral, bacterial, tuberculosis, fungus, and parasites], atopic myelitis, and sarcoidosis). Here, we were initially unable to differentiate demyelinating diseases from a tumor. As the patient demonstrated oligoclonal IgG bands and his clinical symptoms were suggestive for MS versus other demyelinating diseases, steroid pulse therapy was initially performed before surgery.[
Frequency of oligoclonal bands and tumor versus MS/other
Several case reports show that 5.8% of neoplastic lesions of the central nervous system (CNS) may produce oligoclonal IgG bands as do 9.3% of patients with paraneoplastic syndromes.[
Differential diagnosis of intramedullary spinal cord germinoma versus MS with IgG antibodies
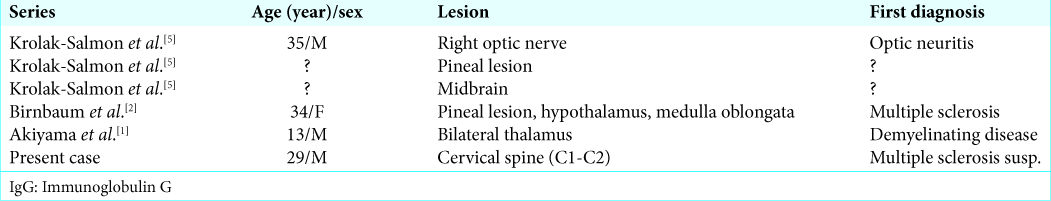
Intramedullary spinal cord germinoma is rare and has been reported in just 16 primary cases.[
CONCLUSION
Here, we report an intramedullary cervical spine germinoma that represented a drop lesion from an original intracranial tumor. Notably, the tumor produced oligoclonal IgG bands in the CSF making it difficult to differentiate between metastatic germinoma and MS.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
References
1. Akiyama Y, Suzuki H, Mikuni N. Germinoma mimicking tumefactive demyelinating disease in pediatric patients. Pediatr Neurosurg. 2016. 51: 149-53
2. Birnbaum T, Pellkofer H, Buettner U. Intracranial germinoma clinically mimicking chronic progressive multiple sclerosis. J Neurol. 2008. 255: 775-6
3. Cohen O, Biran I, Steiner I. Cerebrospinal fluid oligoclonal igG bands in patients with spinal arteriovenous malformation and structural central nervous system lesions. Arch Neurol. 2000. 57: 553-7
4. Kinoshita Y, Akatsuka K, Ohtake M, Kamitani H, Watanabe T. Primary intramedullary spinal cord germinoma. Neurol Med Chir (Tokyo). 2010. 50: 592-4
5. Krolak-Salmon P, Androdias G, Honnorat J, Caudie C, Bret P, Hernette D. Beware of optic neuritis!. Lancet Neurol. 2002. 1: 516-7
6. Miller JR, Burke AM, Bever CT. Occurrence of oligoclonal bands in multiple sclerosis and other CNS diseases. Ann Neurol. 1983. 13: 53-8
7. Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald criteria”. Ann Neurol. 2005. 58: 840-6
8. Psimaras D, Carpentier AF, Rossi C. PNS Euronetwork. Cerebrospinal fluid study in paraneoplastic syndromes. J Neurol Neurosurg Psychiatry. 2010. 81: 42-5