- Department of Neurosurgery, Santa Marcelina Hospital, Sao Paulo, Brazil.
DOI:10.25259/SNI_484_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Daniella Brito Rodrigues, Anderson Batista Rodrigues, Joao Welberthon Matos Queiroz, Murillo Cunegatto Braga, William Seiti Kita, Ricardo Henrique Doria Netto, Rafael Wilson de Souza, Paulo Roberto Napoli, Allexsandro Aparecido Alvarenga Nascimento Faria de Luna. Intramedullary spinal schistosomiasis in a child with acute myelopathy: A case report. 06-Nov-2020;11:371
How to cite this URL: Daniella Brito Rodrigues, Anderson Batista Rodrigues, Joao Welberthon Matos Queiroz, Murillo Cunegatto Braga, William Seiti Kita, Ricardo Henrique Doria Netto, Rafael Wilson de Souza, Paulo Roberto Napoli, Allexsandro Aparecido Alvarenga Nascimento Faria de Luna. Intramedullary spinal schistosomiasis in a child with acute myelopathy: A case report. 06-Nov-2020;11:371. Available from: https://surgicalneurologyint.com/surgicalint-articles/10378/
Abstract
Background: Neuroschistosomiasis is defined as an infection of the nervous system caused by Schistosoma mansoni. Neuroschistosomiasis is an important differential diagnostic consideration in pediatric patients presenting with myelopathy. Surgical excision combined with antiparasitic drugs typically provides a satisfactory outcome and often results in neurological recovery.
Case Description: A 4-year-old child presented with acute and progressive myelopathy. A thoracolumbar magnetic resonance image revealed a T12-L2 conus medullaris mass that was isointense on T1 and hyperintense on T2 (with an extensive syringomyelia at the thoracic spinal cord) and showed enhanced heterogeneity with gadolinium. The lesion was excised through T12-L2 laminotomy. Intraoperatively, the tumor appeared reddish and infiltrative. The frozen section suggested a granulomatous process, while the final pathology confirmed conus medullaris schistosomiasis.
Conclusion: Schistosomal myeloradiculopathy should be considered among the different diagnosis in children presenting with lower thoracic region, conus medullaris, and/or cauda equina infiltrative spinal masses.
Keywords: Conus medullaris, Myelopathy, Neuroschistosomiasis, Schistosomiasis, Spinal
INTRODUCTION
Rarely, neuroschistosomiasis presents a parasitic infection of the lower thoracic area, conus medullaris, and/or cauda equina. Notably, schistosomiasis is endemic to South America, Africa, and certain Asian areas and infects at least 200 million people worldwide.[
Typically, the infection occurs secondary to exposure to water that contains snails with cercariae. The cercariae penetrate the skin and travel through the venous system in which they become adult worms that are capable of releasing eggs. Once in the bloodstream, they are frequently deposited in the gastrointestinal or urinary tract and also rarely in the central nervous system.[
As the parasite reaches the spinal cord, symptoms may appear secondary to the resultant immune response and/ or inflammatory process.[
CASE REPORT
A 4-year-old autistic male from São Paulo presented with a 1-month history of a progressive paraparesis associated with fecal incontinence and urinary retention.
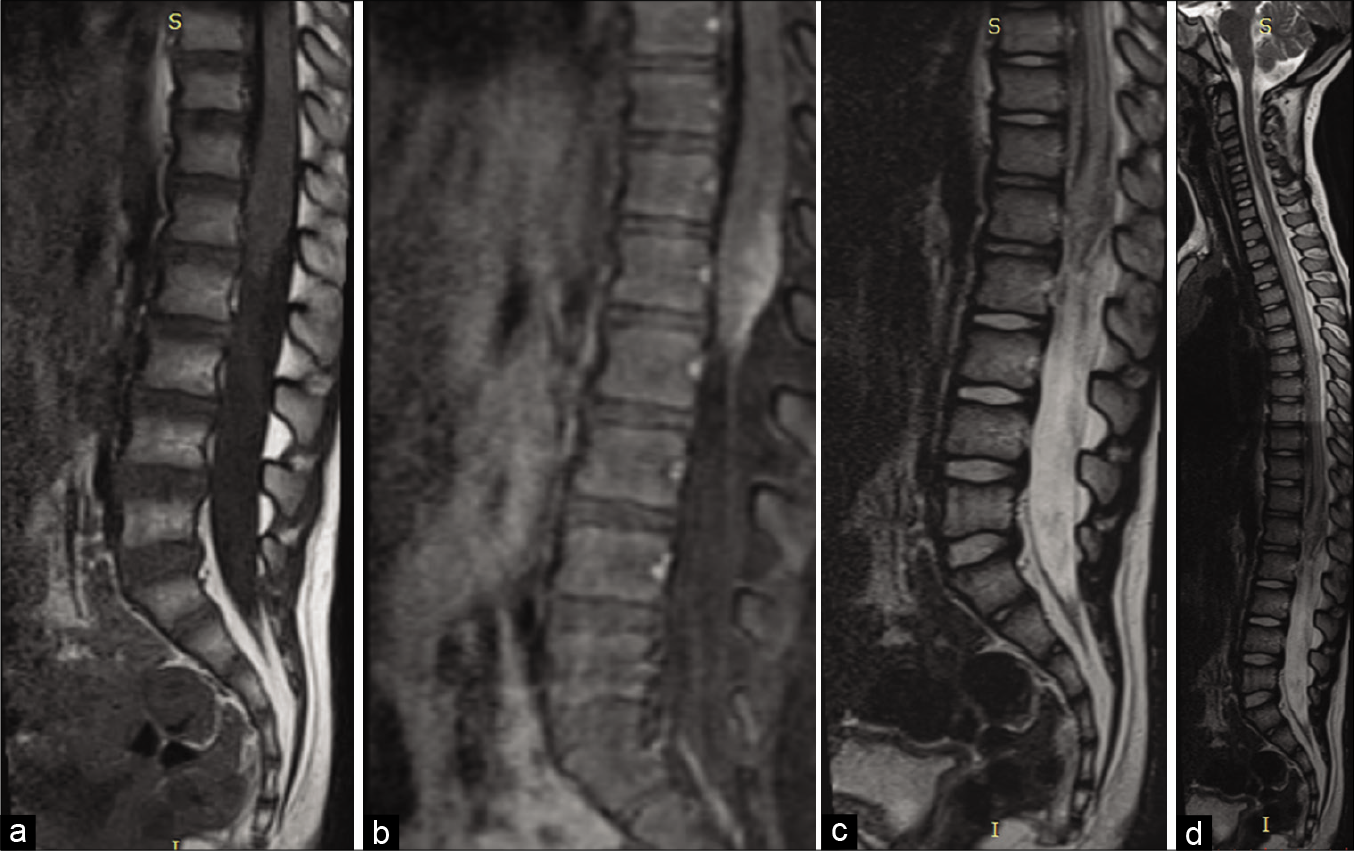
On examination, he was alert, the cranial nerves were intact, and he exhibited a Grade 3/5 motor paraparesis with urinary retention. The thoracolumbar magnetic resonance image (MRI) showed a conus medullaris mass; T2-weighted images demonstrated a T9-T12 syrinx, while the enhanced T1 study showed a heterogeneously enhancing mass [
Figure 1:
Preoperative magnetic resonance imaging (MRI). (a) Sagittal view of the lumbosacral spine T1-weighted without contrast showing an isointense signal lesion in the conus medullaris. (b) MRI sagittal view of the T2-weighted lumbosacral spine showing hyperintense conus medullaris signal. (c) Sagittal MRI of the lumbosacral T1-weighted spine after gadolinium injection, heterogeneous contrast enhancing in conus medullaris, an apparently infiltrative lesion. (d) Sagittal section of the dorsal column and lumbosacral showing extensive syringomyelia in the thoracic spinal cord.
Surgery and outcome
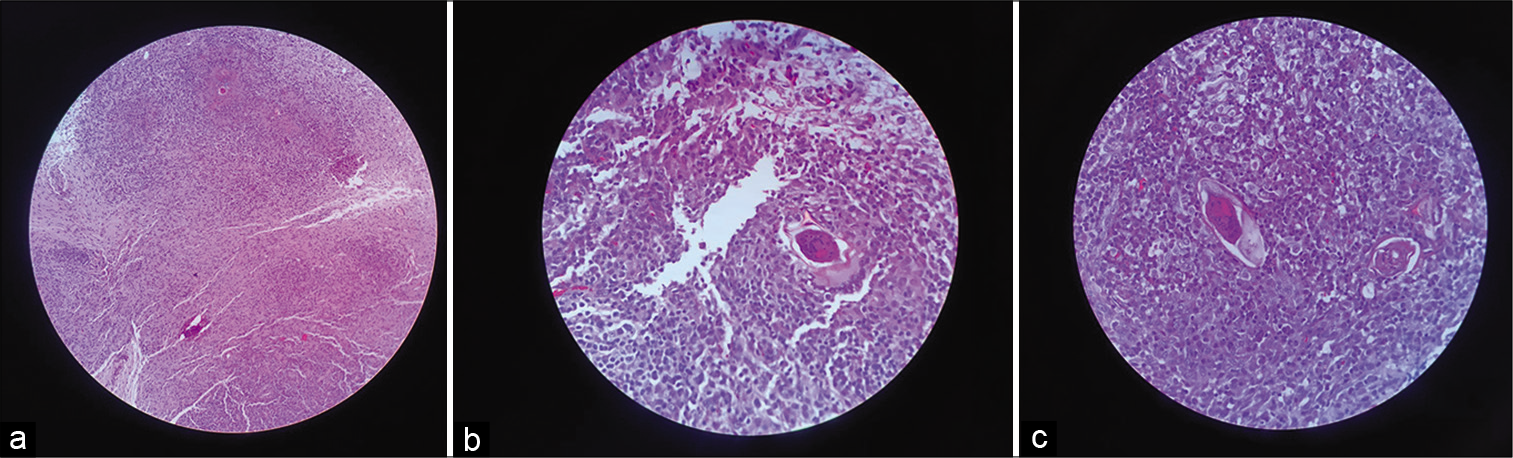
When the T12-L2 laminectomy was performed, the lesion appeared reddish and infiltrative, simulating a high-grade glioma. The intraoperative frozen sections revealed a chronic granulomatous infectious process. The patient was discharged 5 days later with the same preoperative paraparesis; when the final pathology confirmed conus medullaris schistosomiasis, the patient underwent subsequent treatment with praziquantel (20 mg/kg) [
Figure 2:
(a-c) Hematoxylin and eosin (H&E) staining showing numerous granulomatous fragments of fibrotic and neural tissue and lymphomononuclear inflammatory infiltrate rich in eosinophils forming granulomatous nodules with necrotic center enclosing parasites with the characteristic spicula of consistent in appearance with Schistosoma mansoni.
DISCUSSION
In Brazil, 5.6% of inflammatory myelopathy can be attributed to Schistosoma mansoni infection; it must be included among the differential diagnostic considerations for children presenting with acute paraplegia.[
Laboratory studies
Several laboratory tests can assist in establishing the diagnosis of spinal schistosomiasis.[
MRI
On MRI, neuroschistosomal infection may mimic intramedullary tumors.[
Surgery
Biopsy and/or resection remain the gold standard for establishing the diagnosis of spinal schistosomiasis. However, these can be avoided if there is other sufficient confirmatory evidence.[
Drug protocol to treat neuroschistosomiasis
The drug protocol for treating neuroschistosomiasis includes praziquantel with corticosteroids; patients on this regimen typically show neurological improvement in 50.0%–60.3% of cases.[
Outcome
Complete recovery from neuroschistosomiasis is seen in only 30% of S. mansoni cases; residual myeloradiculopathy, and other neurological complications attributed to this disease are common.[
CONCLUSION
Schistosomal myeloradiculopathy should be considered in children with infiltrative lower thoracic, conus medullaris, and/or cauda equina lesions who present with myelopathy particularly in endemic areas.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Camargo ST, Dantas FR, Teixeira AL. Schistosomal myelopathy mimicking spinal cord neoplasm. Scand J Infect Dis. 2005. 37: 365-98
2. Kim AH, Maher CO, Smith ER. Lumbar intramedullary spinal schistosomiasis presenting as progressive paraparesis: Case report. Neurosurgery. 2006. 58: E996
3. Labeodan AO, Sur M. Intramedullary schistosomiasis. Pediatr Neurosurg. 2003. 39: 14-6
4. Lighter J, Kim M, Krasinski K. Intramedullary schistosomiasis presenting in an adolescent with prolonged intermittent back pain. Pediatr Neurol. 2008. 39: 44-7
5. Mikulich O, Chaila E, Crotty JM, Watts M. Spinal cord schistosomiasis presenting as a spinal cord syndrome. BMJ Case Rep. 2013. 2013: bcr2013200229
6. Nascimento-Carvalho CM, Moreto-Carvalho AO. Neuroschistosomiasis due to Schistosoma mansoni: A review of pathogenesis, clinical syndromes and diagnostic approaches. Rev Inst Med Trop São Paulo. 2005. 47: 179-84
7. Pappamikail L, Fernandes P, Gonçalves C. Medullary schistosomiasis. Surg Neurol Int. 2014. 5: 66
8. Paz JA, Valente M, Casella EB, Marques-Dias MJ. Spinal cord schistosomiasis in children: Analysis of seven cases. Arq Neuropsiquiatr. 2002. 60: 224-30
9. Saleem S, Belal AI, El-Ghandour NM. Spinal cord schistosomiasis: MR imaging appearance with surgical and pathologic correlation. AJNR Am J Neuroradiol. 2005. 26: 1646-54
10. Salim AD, Arbab MA, El Hassan LA, El Hassan AM. Schistosomiasis of the spinal cord: Report of 5 cases from Sudan. East Mediterr Health J. 2012. 18: 294-7