- Department of Neurosurgery, University of California Los Angeles (UCLA), Los Angeles, California, United States
- Department of Neurosurgery, University of Texas Southwestern, Dallas, Texas, United States
- Department of Neurosurgery, Harbor (UCLA) Medical Center, Torrance, California, United States.
- Department of Pathology, Harbor (UCLA) Medical Center, Torrance, California, United States.
- Department of Radiology, Harbor (UCLA) Medical Center, Torrance, California, United States.
Correspondence Address:
Isaac Yang, Department of Neurosurgery, University of California Los Angeles(UCLA), Los Angeles, California, United States.
DOI:10.25259/SNI_683_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sophie F. Peeters1, Lauren Uhr1, Srinivas Chivukula2, Richard Everson1, Duc Duong3, Duncan McBride1, Won Kim1, Marcia Cornford4, Anton Mlikotic5, Isaac Yang1. Intraoperative discovery of a radiographically occult subependymoma obstructing the obex in a patient with a Chiari malformation – A rare case. 05-Jan-2024;15:4
How to cite this URL: Sophie F. Peeters1, Lauren Uhr1, Srinivas Chivukula2, Richard Everson1, Duc Duong3, Duncan McBride1, Won Kim1, Marcia Cornford4, Anton Mlikotic5, Isaac Yang1. Intraoperative discovery of a radiographically occult subependymoma obstructing the obex in a patient with a Chiari malformation – A rare case. 05-Jan-2024;15:4. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12700
Abstract
Background: Chiari (type I) malformations are typically congenital. Occasionally, however, tonsillar herniation can arise secondary to cerebrospinal fluid leakage, posterior fossa or intraventricular mass lesions, or other etiologies. We present the first-ever case of an intramedullary subependymoma at the cervicomedullary junction associated with vertebral bone abnormalities and an acquired secondary Chiari malformation.
Case Description: A 60-year-old woman presented with a 3-year history of occipital, tussive headaches. Preoperative imaging was negative for mass lesions but demonstrated a Chiari malformation. She was recommended posterior fossa decompression with tonsillar shrinkage. During surgery, an intramedullary mass was incidentally observed, obstructing the obex at the cervicomedullary junction. Histopathological analysis of the resected lesion revealed a diagnosis of subependymoma.
Conclusion: Subependymomas can sometimes present a diagnostic challenge due to their subtle appearance in neuroimaging. Only rarely are such masses associated with an acquired Chiari malformation. No such case has previously been reported. We present a literature review on acquired Chiari malformations and discuss their management.
Keywords: Atlanto-occipital assimilation, Chiari malformation, Radiographically occult, Subependymoma, Vertebral anomaly
INTRODUCTION
The type I Chiari malformation is characterized by a caudal displacement of the cerebellar tonsils through the foramen magnum into the cervical canal of more than 5 mm. This malformation is generally congenital, affecting approximately 1 in 1000 births. Often asymptomatic in childhood, it can present as tussive headaches or neck pain in young adults. There are, however, cases of acquired Chiari malformations that have been described to arise secondarily, such as lumbar shunting procedures or spontaneous cerebrospinal fluid (CSF) leakage.[
Subependymomas are a benign, indolent subtype of ependymomas that develop from glial cells that line the ventricles and the central spinal canal.[
CASE DESCRIPTION
Clinical presentation
A 60-year-old woman with a medical history of gastroesophageal reflux disease, anemia, and knee osteoarthritis status post bilateral knee replacements presented with occipital, tussive headaches for three years. She also noted intermittent subjective left upper extremity weakness and bilateral upper extremity numbness. She denied bowel or bladder issues, vision changes, nausea, or vomiting. Her husband noted nighttime snoring without apneic episodes.
On physical examination, her cranial nerves were grossly intact, and her motor strength was 5/5 in all extremities with intact sensation to light touch. There were no signs of spasticity on examination, with no long tract signs. Cerebellar examination and gait were within normal limits.
Diagnostic results
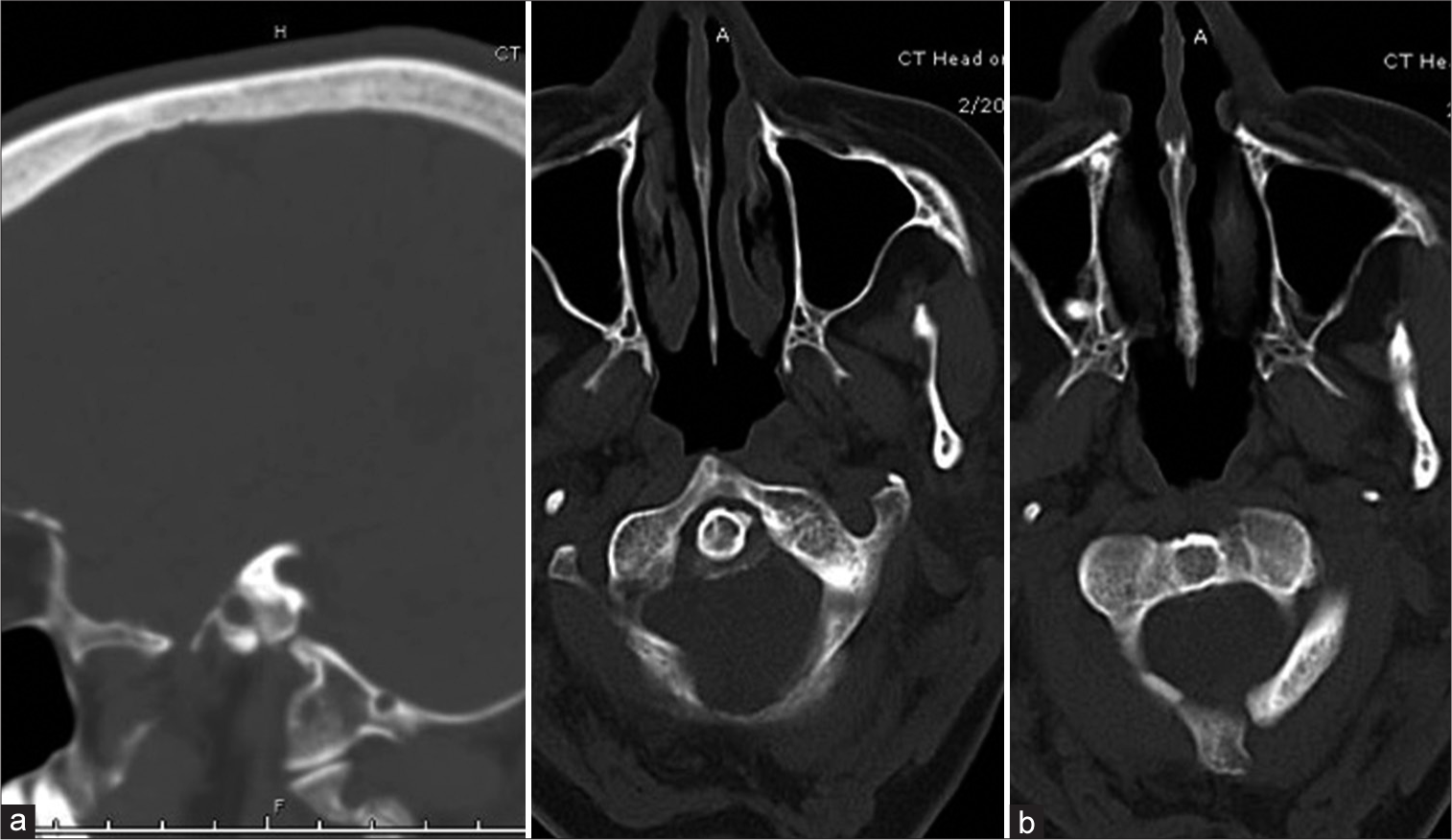
Computed tomography (CT) of the brain demonstrated crowding at the craniocervical junction with downward displacement of the cerebellar tonsils and a syrinx of the visualized upper cervical cord. Furthermore, there was evidence on CT of partial occipitoatlantal assimilation as well as basilar invagination [
Treatment
Given that the patient was symptomatic from tonsillar descent, she was recommended surgical intervention. She underwent a suboccipital craniectomy and a C1 laminectomy for intradural decompression. The C1 lamina was noted to be atretic on one side and occipitalized on the other. It was resected to 1.5 cm from midline bilaterally. The suboccipital fossa bone and dura were noted to be thinned. The cerebellar tonsils were downwardly displaced and immobile. They were shrunken through subpial coagulation and partially resected. Deep to the cerebellar tonsils, a gray, semi-firm, and intramedullary lesion was noted obstructing the obex, tracking both cephalad and caudad within the central canal [
Video 1
Postoperative outcome and follow-up
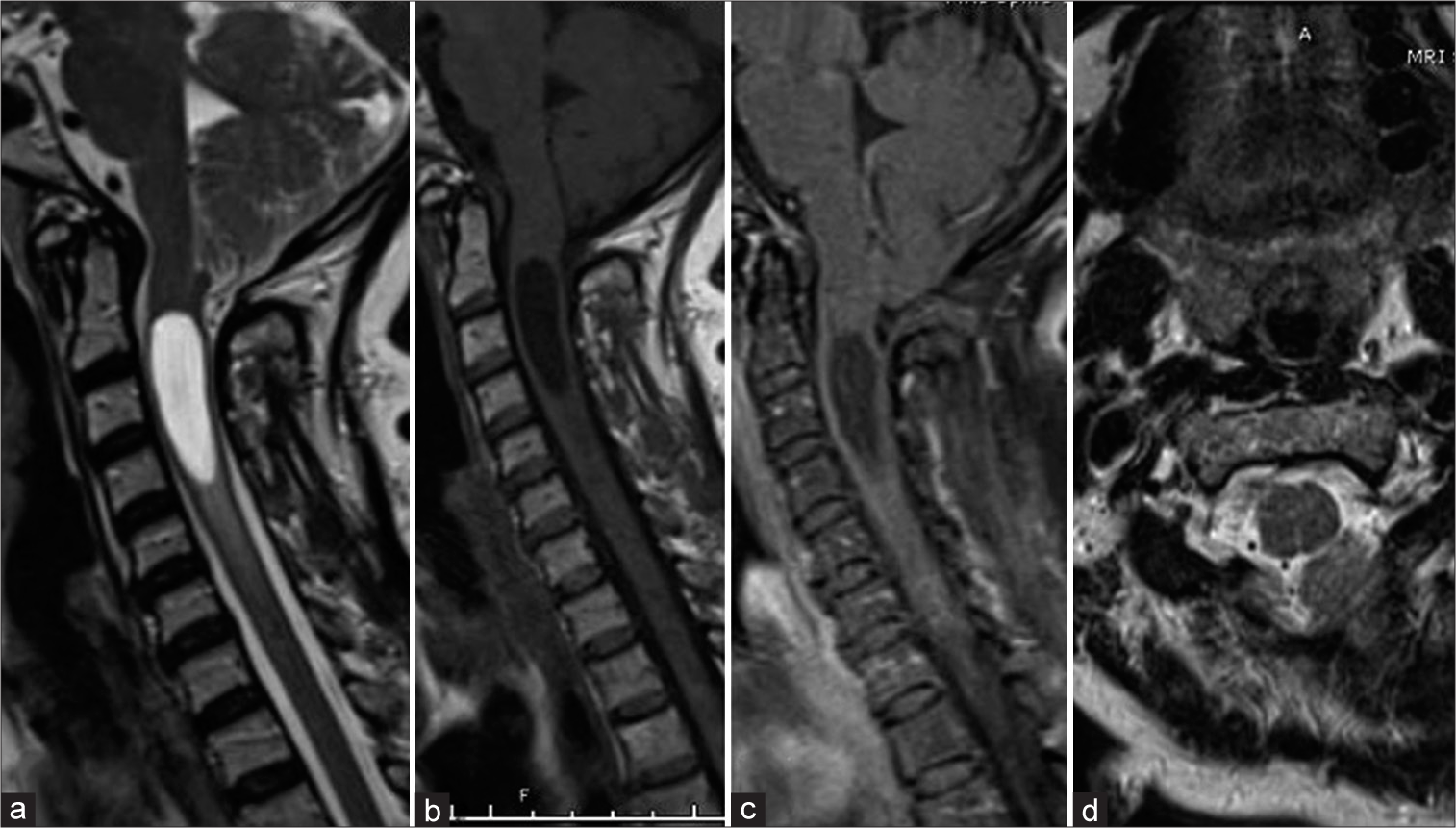
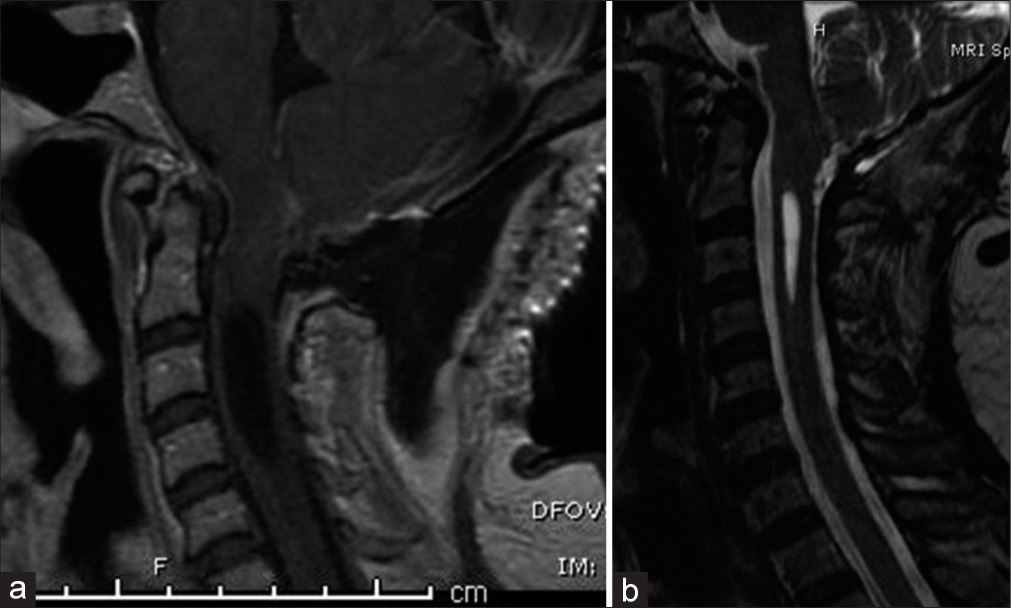
Postoperatively, the patient reported left upper extremity paresthesias, new blurry vision, dizziness, and intermittent diplopia. Ophthalmology was consulted; both visual and extraocular symptoms were thought to be secondary to cerebellar disturbance from recent surgery. Dexamethasone therapy was started with moderate improvement of her visual symptoms. A postoperative MRI of the brain soon after surgery demonstrated decompression of the suboccipital region and expected postoperative changes [
Figure 3:
(a) Immediate postoperative magnetic resonance imaging (MRI) sagittal post-contrast sequence demonstrating no visible lesion in the cervicomedullary junction and (b) delayed 4-month postoperative T2-weighted MRI sagittal view with a significant reduction in the size of the cervical syringomyelia.
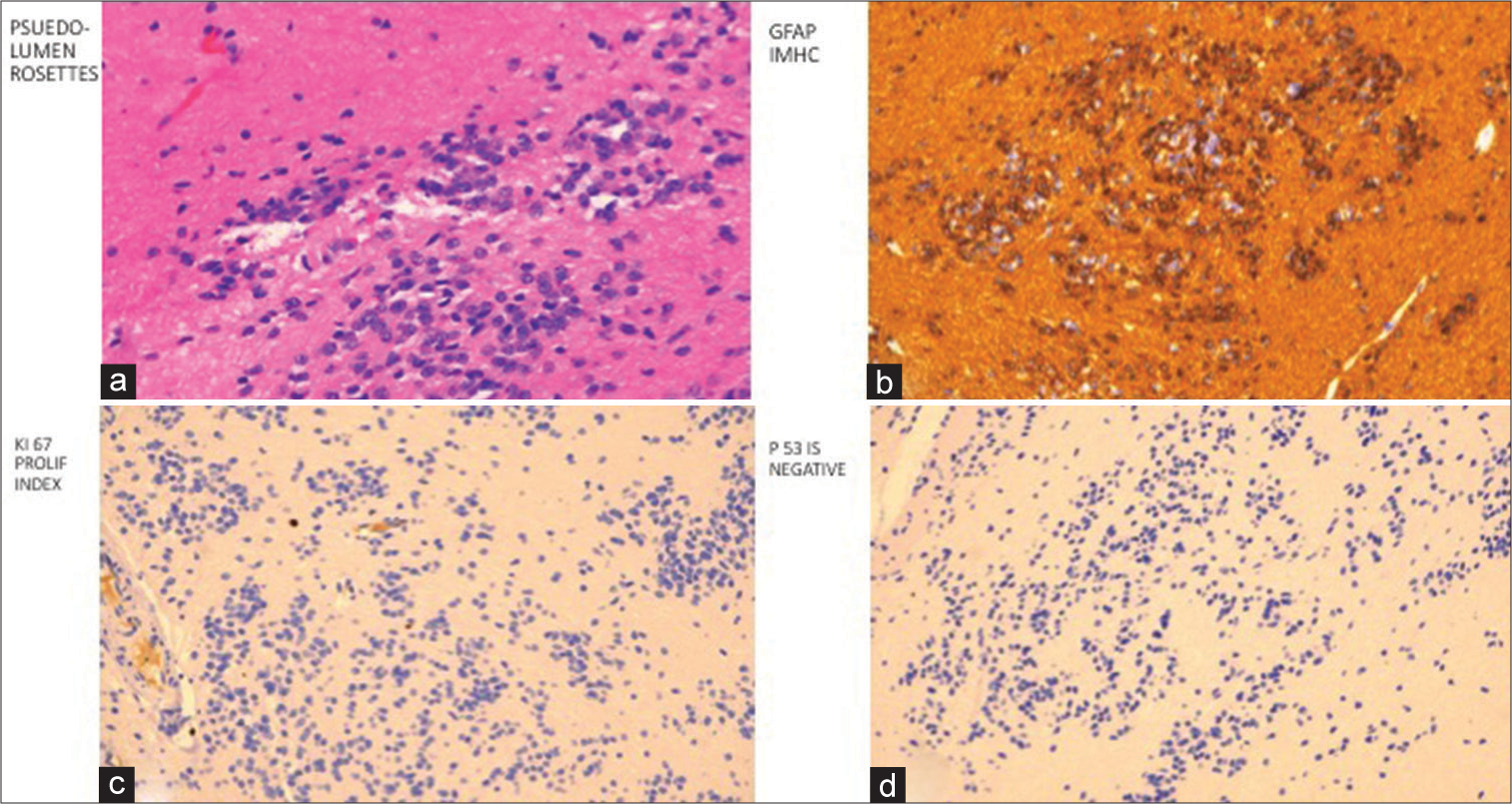
Pathological diagnosis
The final pathologic diagnosis of the posterior fossa mass was a World Health Organization (WHO) Grade 1 subependymoma. Histopathological review demonstrated pseudo-lumen rosettes and a nodular pattern [
DISCUSSION
Subependymomas, a subtype of ependymomas, are rare, benign tumors of the central nervous system that is histologically classified as low grade (WHO Grade 1). They represent between 0.07 and 0.7% of all intracranial tumors and arise from bipotent subependymal glial precursor cells.[
Acquired Chiari malformations, defined as arising postnatally, are radiographically and clinically indistinguishable from congenital Chiari I malformations. In the literature, cases of acquired malformations have been associated with lumboperitoneal shunting, spontaneous CSF leak, and lumbar puncture.[
In our case, there was no appreciable lesion on preoperative imaging (CT or MRI), as confirmed by neuroradiologists, who reviewed the imaging again retrospectively. Imaging characteristics of intracranial subependymomas vary slightly by their location.[
Subependymomas tend to be slow-growing and indolent, evidenced by lower MIB-1 indices than other ependymal tumors, although their clinical course can vary widely based on location, size, and growth rate.[
In the cases of a mass lesion associated with an acquired Chiari malformation and syringomyelia, resection of the lesion is generally necessary to restore normal pulsatile CSF flow through the obex and achieve symptomatic improvement. Whether the traditional posterior fossa decompression through suboccipital craniectomy and possible C1 laminectomy is necessary in these cases remains unclear.[
Surgical management is the first-line treatment for symptomatic subependymomas.[
As the recurrence rate is extremely low, there is limited evidence regarding the management of recurrent subependymomas. Ecker and Pollock reported a case of a recurrent fourth ventricular subependymoma that after multiple subtotal resections was ultimately managed with stereotactic radiosurgery with no further recurrence at 54 month follow-up.[
Finally, our patient also had associated vertebral bone abnormalities (C1 atresia and atlanto-occipital assimilation). This finding itself is exceedingly rare. Morselli et al. reported a case of intradural extramedullary Grade II ependymoma with C1 partial agenesis.[
CONCLUSION
Acquired Chiari malformations can be due to CSF shunting or leaking or, less commonly, secondary to a space-occupying lesion in the cervicomedullary junction. We present the only case of an acquired Chiari malformation secondary to an intramedullary subependymoma in the cervicomedullary junction, radiographically occult and incidentally discovered during surgery. Subependymomas can be challenging to diagnose on imaging, as illustrated by our case and another by Varma et al., both unnoticed on preoperative radiologic imaging. Most of these lesions are associated with syringomyelia, which nearly always resolves (or at least decreases), as do the neurologic symptoms after removal of the lesion. Therefore, suboccipital craniectomy and shrinkage of the cerebellar tonsils are often unnecessary in this patient population with acquired Chiari malformations. Finally, as subependymomas can recur or cause drop metastases in the central nervous system, close follow-up is required for these patients, especially following subtotal resection.
Ethical approval
The research/study complied with the Helsinki Declaration of 1964.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Videos available on:
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Atkinson JL, Weinshenker BG, Miller GM, Piepgras DG, Mokri B. Acquired Chiari I malformation secondary to spontaneous spinal cerebrospinal fluid leakage and chronic intracranial hypotension syndrome in seven cases. J Neurosurg. 1998. 88: 237-42
2. Bi Z, Ren X, Zhang J, Jia W. Clinical, radiological, and pathological features in 43 cases of intracranial subependymoma. J Neurosurg. 2015. 122: 49-60
3. Caldarelli M, Novegno F, Di Rocco C. A late complication of CSF shunting: Acquired Chiari I malformation. Childs Nerv Syst. 2009. 25: 443-52
4. Chandra PS, Gupta A, Mishra NK, Mehta VS. Association of craniovertebral and upper cervical anomalies with dermoid and epidermoid cysts: Report of four cases. Neurosurgery. 2005. 56: E1155
5. Chiechi MV, Smirniotopoulos JG, Jones RV. Intracranial subependymomas: CT and MR imaging features in 24 cases. AJR Am J Roentgenol. 1995. 165: 1245-50
6. Ecker RD, Pollock BE. Recurrent subependymoma treated with radiosurgery. Stereotact Funct Neurosurg. 2004. 82: 58-60
7. Jain A, Amin AG, Jain P, Burger P, Jallo GI, Lim M. Subependymoma: Clinical features and surgical outcomes. Neurol Res. 2012. 34: 677-84
8. Kandenwein JA, Bostroem A, Feuss M, Pietsch T, Simon M. Surgical management of intracranial subependymomas. Acta Neurochir (Wien). 2011. 153: 1469-75
9. Kong LY, Wei J, Haider AS, Liebelt BD, Ling X, Conrad CA. Therapeutic targets in subependymoma. J Neuroimmunol. 2014. 277: 168-75
10. Laxton AW, Shannon P, Nag S, Farb RI, Bernstein M. Rapid expansion of a previously asymptomatic subependymoma. Case report. J Neurosurg. 2005. 103: 1084-7
11. Morselli C, Ruggeri AG, Pichierri A, Marotta N, Anzidei M, Delfini R. Intradural extramedullary primary ependymoma of the craniocervical junction combined with C1 partial agenesis: Case report and review of the literature. World Neurosurg. 2015. 84: 6.e1-6
12. Ortiz-Reyes R, Dragovic L, Eriksson A. Sudden unexpected death resulting from previously nonsymptomatic subependymoma. Am J Forensic Med Pathol. 2002. 23: 63-7
13. Owler BK, Halmagyi GM, Brennan J, Besser M. Syringomyelia with Chiari malformation; 3 unusual cases with implications for pathogenesis. Acta Neurochir (Wien). 2004. 146: 1137-43
14. Payner TD, Prenger E, Berger TS, Crone KR. Acquired Chiari malformations: Incidence, diagnosis, and management. Neurosurgery. 1994. 34: 429-34
15. Piatt JH, D’Agostino A. The Chiari II malformation: Lesions discovered within the fourth ventricle. Pediatr Neurosurg. 1999. 30: 79-85
16. Prayson RA, Suh JH. Subependymomas: Clinicopathologic study of 14 tumors, including comparative MIB-1 immunohistochemical analysis with other ependymal neoplasms. Arch Pathol Lab Med. 1999. 123: 306-9
17. Reni M, Gatta G, Mazza E, Vecht C. Ependymoma. Crit Rev Oncol Hematol. 2007. 63: 81-9
18. Seol HJ, Hwang SK, Choi YL, Chi JG, Jung HW. A case of recurrent subependymoma with subependymal seeding: Case report. J Neurooncol. 2003. 62: 315-20
19. Varma A, Giraldi D, Mills S, Brodbelt AR, Jenkinson MD. Surgical management and long-term outcome of intracranial subependymoma. Acta Neurochir (Wien). 2018. 160: 1793-9
20. Wang J, Alotaibi NM, Samuel N, Ibrahim GM, Fallah A, Cusimano MD. Acquired Chiari malformation and syringomyelia secondary to space-occupying lesions: A systematic review. World Neurosurg. 2017. 98: 800-8.e2
21. Wistler O, Schiffer D, editors. Subependymoma: Tumors of the nervous system. Pathology and Genetics Tumours of the Nervous System. Lyon: IARC Press; 2000. p. 80-1